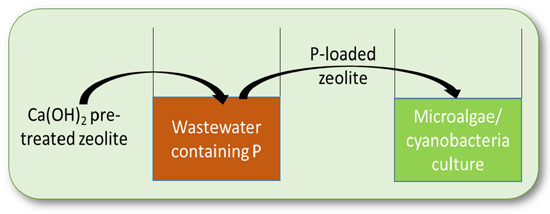
Cultivation of Chlorella vulgaris and Arthrospira platensis with Recovered Phosphorus from Wastewater by Means of Zeolite Sorption
Abstract
:1. Introduction
2. Results
2.1. Desorption of Phosphorus


2.2. Cultivation of C. vulgaris and A. platensis with P-Loaded Zeolite
2.2.1. Cultivation with 0.15–1.00 g/L P-Loaded Zeolite




2.2.2. Cultivation with 5 g/L P-Loaded Zeolite
| P-Loaded Zeolite | Intracellular P (mg-P/g) | Carbohydrates (% Dry Biomass) | Proteins (% Dry Biomass) | Lipids (% Dry Biomass) | ||||
|---|---|---|---|---|---|---|---|---|
| C. vulgaris | A. platensis | C. vulgaris | A. platensis | C. vulgaris | A. platensis | C. vulgaris | A. platensis | |
| 0.15 | 3.7 ± 0.3 | 1.9 ± 0.2 | 31 ± 2 | 70 ± 3 | 16 ± 2 | 19 ± 2 | 11 ± 2 | 7.2 ± 0.5 |
| 0.2 | 4.0 ± 0.5 | 1.9 ± 0.2 | 28 ± 1 | 71 ± 3 | 16 ± 1 | 21 ± 2 | 13 ± 2 | 5 ± 2 |
| 0.25 | 4.9 ± 0.2 | 1.78 ± 0.07 | 36 ± 2 | 71 ± 2 | 15.8 ± 0.4 | 18 ± 1 | 12 ± 4 | 5.9 ± 0.8 |
| 0.35 | 5.6 ± 0.6 | 1.7 ± 0.2 | 37 ± 1 | 63 ± 4 | 16 ± 2 | 16 ± 1 | 16 ± 2 | 6.0 ± 0.5 |
| 0.5 | 5.5 ± 0.5 | 1.8 ± 0.1 | 42 ± 3 | 61 ± 8 | 16 ± 2 | 18.5 ± 0.6 | 19 ± 2 | 5.0 ± 0.8 |
| 1 | 8.5 ± 0.7 | 2.1 ± 0.2 | 48 ± 5 | 52 ± 12 | 21 ± 2 | 29 ± 2 | 24 ± 5 | 12.5 ± 0.6 |
| Control (with K2HPO4) | 7.8 ± 0.6 | 6.2 ± 0.4 | 43 ± 1 | 14 ± 2 | 25 ± 1 | 32 ± 1 | 21 ± 2 | 8.8 ± 0.8 |
| Species | Culture | Intracellular P (mg-P/g) | Carbohydrates (% Dry Biomass) | Proteins (% Dry Biomass) | Lipids (% Dry Biomass) | Chlorophyll α | Chlorophyll β | Carotenoids |
|---|---|---|---|---|---|---|---|---|
| C. vulgaris | 5 g/L | 9.4 ± 0.3 | 35.9 ± 0.8 | 22 ± 1 | 25 ± 2 | 2.1 ± 0.3 | 0.5 ± 0.1 | 0.60 ± 0.05 |
| Control | 13 ± 2 | 40 ± 1 | 35 ± 4 | 18 ± 1 | 2.17 ± 0.06 | 0.56 ± 0.03 | 0.62 ± 0.02 | |
| A. platensis | 5 g/L | 6.3 ± 0.5 | 20 ± 1 | 53 ± 2 | 12.4 ± 0.6 | 1.11 ± 0.07 | – | 0.32 ± 0.01 |
| Control | 6.8 ± 0.5 | 18 ± 1 | 53 ± 3 | 10.8 ± 0.5 | 1.07 ± 0.06 | – | 0.32 ± 0.02 |

3. Discussion
4. Experimental Section
4.1. Microorganisms
4.2. Experimental Set-Up
4.2.1. Enrichment of Zeolite with P
4.2.2. P Desorption Kinetics
4.2.3. P-Recovery Calculations
4.2.4. Cultivation of C. vulgaris and A. platensis
4.3. Analytical Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Grobbelaar, J.U. Algal niutrition. Mineral nutrition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 97–115. [Google Scholar]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Depraetere, O.; Foubert, I.; Muylaert, K. Decolorisation of piggery wastewater to stimulate the production of Arthrospira platensis. Bioresour. Technol. 2013, 148, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jin, H.-F.; Lim, B.-R.; Park, K.-Y.; Lee, K. Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp. Bioresour. Technol. 2010, 101, 8649–8657. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Vandamme, D.; Muylaert, K. Using natural zeolite for ammonia sorption from wastewater and as nitrogen releaser for the cultivation of Arthrospira platensis. Bioresour. Technol. 2014, 155, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Tokuşoglu, Ö.; Ünal, M.K. Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar]
- Powell, N.; Shilton, A.N.; Pratt, S.; Chisti, Y. Factors influencing luxury uptake of phosphorus by microalgae in waste stabilization ponds. Environ. Sci. Technol. 2008, 42, 5958–5962. [Google Scholar] [CrossRef] [PubMed]
- Beardall, J.; Roberts, S.; Raven, J.A. Regulation of inorganic carbon acquisition by phosphorus limitation in the green alga Chlorella emersonii. Can. J. Bot. 2005, 83, 859–864. [Google Scholar] [CrossRef]
- Belay, A. Spirulina (Arthrospira): Production and quality assurance. In Spirulina in Human Nutrition and Health; Gershwin, M.E., Belay, A., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 1–25. [Google Scholar]
- Markou, G.; Chatzipavlidis, I.; Georgakakis, D. Carbohydrates production and bio-flocculation characteristics in cultures of Arthrospira (spirulina) platensis: Improvements through phosphorus limitation process. BioEnergy Res. 2012, 5, 915–925. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae: Towards meeting Advanced Fuel Standards. In Advanced Biofuels and Bioproducts; Lee, J.W., Ed.; Springer: New York, NY, USA, 2013; pp. 553–599. [Google Scholar]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jin, X.; Yao, Y.; Li, L.; Wu, F. Effects of biological activity, light, temperature and oxygen on phosphorus release processes at the sediment and water interface of taihu lake, china. Water Res. 2008, 42, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Sundbäck, K.; Miles, A.; Goeransson, E. Nitrogen fluxes, denitrification and the role of microphytobenthos in microtidal shallow-water sediments: An annual study. Mar. Ecol. Prog. Ser. 2000, 200, 59–76. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Hamidpour, M.; Afyuni, M.; Kalbasi, M.; Khoshgoftarmanes, A.H.; Inglezakis, V.J. Mobility and plant-availability of Cd(II) and Pb(II) adsorbed on zeolite and bentonite. Appl. Clay Sci. 2010, 48, 342–348. [Google Scholar] [CrossRef]
- He, P.J.; Mao, B.; Shen, C.M.; Shao, L.M.; Lee, D.J.; Chang, J.S. Cultivation of Chlorella vulgaris on wastewater containing high levels of ammonia for biodiesel production. Bioresour. Technol. 2013, 129, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Travieso, L.; Benítez, F.; Sánchez, E.; Borja, R.; Martín, A.; Colmenarejo, M.F. Batch mixed culture of Chlorella vulgaris using settled and diluted piggery waste. Ecol. Eng. 2006, 28, 158–165. [Google Scholar] [CrossRef]
- Mitchell, S.A.; Richmond, A. Optimization of a growth medium for Spirulina based on cattle waste. Biol. Wastes 1988, 25, 41–50. [Google Scholar] [CrossRef]
- Phang, S.M.; Miah, M.S.; Yeoh, B.G.; Hashim, M.A. Spirulina cultivation in digested sago starch factory wastewater. J. Appl. Phycol. 2000, 12, 395–400. [Google Scholar] [CrossRef]
- Eixler, S.; Karsten, U.; Selig, U. Phosphorus storage in Chlorella vulgaris (Trebouxiophyceae, Chlorophyta) cells and its dependence on phosphate supply. Phycologia 2006, 45, 53–60. [Google Scholar] [CrossRef]
- Baldia, S.F.; Fukami, K.; Nishijima, T.; Hata, Y. Growth responses of Spirulina platensis to some physico-chemical factors and the kinetics of phosphorus utilization. Fish. Sci. 1995, 61, 331–335. [Google Scholar] [CrossRef]
- Fitzgerald, G.P.; Nelson, T.C. Extractive and enzymatic analyses for limiting or surplus phosphorus in algae. J. Phycol. 1975, 11, 32–37. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Geider, R.J.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae—Novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Zhang, Q.; Gu, M.; Cong, W. Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J. Appl. Phycol. 2013, 25, 311–318. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Ion exchange of Pb2+, Cu2+, Fe3+, and Cr3+ on natural clinoptilolite: Selectivity determination and influence of acidity on metal uptake. J. Colloid Interface Sci. 2003, 261, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kalló, D. Applications of natural zeolites in water and wastewater treatment. Rev. Mineral. Geochem. 2001, 45, 519–550. [Google Scholar] [CrossRef]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Izard, J.; Limberger, R.J. Rapid screening method for quantitation of bacterial cell lipids from whole cells. J. Microbiol. Methods 2003, 55, 411–418. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Solorzano, L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995; p. 1325. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markou, G.; Depraetere, O.; Vandamme, D.; Muylaert, K. Cultivation of Chlorella vulgaris and Arthrospira platensis with Recovered Phosphorus from Wastewater by Means of Zeolite Sorption. Int. J. Mol. Sci. 2015, 16, 4250-4264. https://doi.org/10.3390/ijms16024250
Markou G, Depraetere O, Vandamme D, Muylaert K. Cultivation of Chlorella vulgaris and Arthrospira platensis with Recovered Phosphorus from Wastewater by Means of Zeolite Sorption. International Journal of Molecular Sciences. 2015; 16(2):4250-4264. https://doi.org/10.3390/ijms16024250
Chicago/Turabian StyleMarkou, Giorgos, Orily Depraetere, Dries Vandamme, and Koenraad Muylaert. 2015. "Cultivation of Chlorella vulgaris and Arthrospira platensis with Recovered Phosphorus from Wastewater by Means of Zeolite Sorption" International Journal of Molecular Sciences 16, no. 2: 4250-4264. https://doi.org/10.3390/ijms16024250
APA StyleMarkou, G., Depraetere, O., Vandamme, D., & Muylaert, K. (2015). Cultivation of Chlorella vulgaris and Arthrospira platensis with Recovered Phosphorus from Wastewater by Means of Zeolite Sorption. International Journal of Molecular Sciences, 16(2), 4250-4264. https://doi.org/10.3390/ijms16024250