A New Series of Kinked Liquid Crystals: 2-(6-Alkoxynaphthalen-2-yl)-6-methoxyquinolines
Abstract
:1. Introduction
2. Results and Discussion
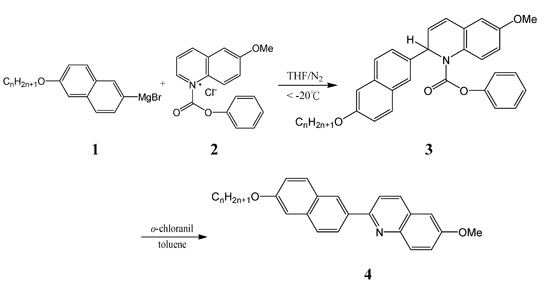
2.1. Synthesis


| Entry (n) | Alkyl Group | Yield a (%) |
|---|---|---|
| 3 | Propyl | 58 |
| 4 | Butyl | 43 |
| 5 | Pentyl | 46 |
| 6 | Hexyl | 52 |
| 7 | Heptyl | 52 |
| 8 | Octyl | 48 |
2.2. Thermotropic Studies
| Compound nO-NpQOMe (n) | Phase Transition Temperatures (°C) and Corresponding Transition Enthalpies (kJ·mol−1) | |
|---|---|---|
| Heating | Cooling | |
| 3 | Cr 213.7(31.81) N 234.2(0.27) I | I 232.6(0.37) N 185.9(27.13) Cr |
| 4 | Cr 205.3(29.51) N 231.6(0.40) I | I 230.1(0.45) N 175.1(25.05) Cr |
| 5 | Cr 196.1(25.71) N 217.7(0.25) I | I 216.3(0.28) N 169.3(23.05) Cr |
| 6 | Cr 192.1(29.48) N 213.9(0.24) I | I 212.2(0.26) N 167.7(27.23) Cr |
| 7 | Cr 187.0(24.95) N 204.4(0.21) I | I 203.0(0.23) N 163.2(21.84) Cr |
| 8 | Cr 184.4(24.94) N 201.0(0.19) I | I 199.4(0.27) N 162.4(23.28) Cr |





3. Experimental Section
3.1. General
3.2. Synthesis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, S. Nematic liquid crystals. In Liquid Crystals: Fundamentals; World Scientific: London, UK, 2002; pp. 92–109. [Google Scholar]
- Oswald, P.; Pieranski, P. Mesogenic Anatomy. In Nematic and Cholesteric Liquid Crystals; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 35–46. [Google Scholar]
- Lauk, U.; Skrabal, P.; Zollinger, H. Substituted 2-phenylnaphthalenes, a new class of nematic liquid crystals. Helv. Chim. Acta 1981, 64, 1847–1848. [Google Scholar] [CrossRef]
- Lauk, U.; Skrabal, P.; Zollinger, H. Mesomophic properties of some 6-n-alkyl-2-(4'-cyanophenyl)naphthalenes. Helv. Chim. Acta 1983, 66, 1574–1575. [Google Scholar] [CrossRef]
- Paraskos, P.A.; Swager, T.M. Effects of desymmetrization on thiophene-based bent-rod mesogens. Chem. Mater. 2002, 14, 4543–4549. [Google Scholar] [CrossRef]
- Madsen, L.A.; Dingemans, T.J.; Nakata, M.; Samulski, E.T. Thermotropic biaxial nematic liquid crystals. Phys. Rev. Lett. 2004, 92. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Yamamoto, T. Synthesis of novel nematic liquid crystals containing 3,3'-dimethyl-2,2'-bipydiyl. Liq. Cryst. 2002, 29, 67–70. [Google Scholar] [CrossRef]
- Leardini, R.; Nanni, D.; Pedulli, G.F.; Tundo, A.; Zanardi, G. Liquid-crystalline quinoline derivatives. Liq. Cryst. 1987, 2, 625–631. [Google Scholar] [CrossRef]
- Yokoyama, A.; Nishiyama, I.; Yoshizawa, A. 6-alkyl-2-(4-alkyloxyphenyl)quinoline: A new smectic C base material. Ferroelectrics 1993, 148, 139–145. [Google Scholar] [CrossRef]
- Zuniga, C.; Bartulin, J.; Muller, H.J.; Schumacher, E.; Taylor, T.R. Synthesis and mesomorphic properties of 2-N-hexyl-6-(4-N-alkoxybenzoyloxy)quinoline. Mol. Cryst. Liq. Cryst. 1991, 206, 131–137. [Google Scholar] [CrossRef]
- Zuniga, C.; Belmar, J.; Parra, M.; Ramirez, A.; Decap, J.; Ros, B.; Serrano, J.L. Synthesis and mesomorphic properties of 6-n-decyloxy-2-[4'-N-alkoxyphenylimino)methyl]quinolines (IV). Liq. Cryst. 1996, 20, 253–260. [Google Scholar] [CrossRef]
- Belmar, J.; Parra, M.; Zuniga, C.; Fuentes, G.; Marcos, M.; Serrano, J.L. Synthesis and mesomorphic properties of 2,6-disubstituted derivatives of quinoline: Amides and esters. Liq. Cryst. 1999, 26, 9–15. [Google Scholar] [CrossRef]
- Eisch, J.J.; Dluzniewski, T. Mechanism of the Skraup and Doebner-von Miller quinoline synthesis: Cyclization of a,b-unsaturated N-aryliminium salts via 1,3-diazetidinium ion intermediates. J. Org. Chem. 1989, 54, 1269–1274. [Google Scholar] [CrossRef]
- Gilchrist, T.L. Six-membered ring compounds with one heteroatom. In Heterocyclic Chemistry; Longman Scientific & Technical: Harlow, Essex, UK, 1985; pp. 270–272. [Google Scholar]
- Lin, H.-C.; Lai, L.-L.; Hsieh, W.-P.; Huang, W.-Y. A novel class of heterocyclic liquid crystals with broad smectic C phase. Liq. Cryst. 1997, 22, 661–667. [Google Scholar] [CrossRef]
- Meth-Cohn, O.; Narine, B.; Tarnowski, B. A versatile new synthesis of quinolines and related fused pyridines. Part II. Tetrahedron Lett. 1979, 33, 3111–3114. [Google Scholar] [CrossRef]
- Lai, L.-L.; Wang, C.-H.; Hsieh, W.P.; Lin, H.-C. Synthesis and characterization of liquid crystalline molecules containing the quinoline unit. Mol. Cryst. Liq. Cryst. 1996, 287, 177–181. [Google Scholar] [CrossRef]
- Sato, K.; Kitayama, H.; Shinjo, K.; Nakamura, S.; Nakamura, K. Liquid Crystal Composition, Liquid Crystal Device Using the Composition, Liquid Crystal Apparatus and Display Method. U.S. Patent 5,948,317, 7 September 1999. [Google Scholar]
- Kosaka, Y.; Takiguchi, T.; Iwaki, T.; Togano, T.; Nakamura, S. Mesomorphic Compound, Liquid Crystal Composition Containing the Compound, Liquid Crystal Device Using the Composition, Liquid Crystal Apparatus and Display Method. U.S. Patent 5,695,684, 9 December 1997. [Google Scholar]
- Yokoyama, A.; Yoshizawa, A.; Hirai, T. New Phenylquinoline Compound and Liquid Crystal Composition Containing the Same. Japan Patent 1992-316555, 6 November 1992. [Google Scholar]
- Chia, W.L.; Liao, K.H.; Ho, C.I. Synthesis and mesomorphic properties on the series of 2-(4-alkylphenyl)-6-methylquinolines and 2-(4-alkoxyphenyl)-6-methylquinolines. Liq. Cryst. 2009, 36, 557–563. [Google Scholar] [CrossRef]
- Chia, W.-L.; Chang, C.H. Synthesis and mesomorphic studies of a series of liquid crystalline 2-(4-alkylphenyl)-6-methoxyquinolines. Mol. Cryst. Liq. Cryst. 2009, 506, 47–55. [Google Scholar] [CrossRef]
- Chia, W.L.; Ye, F.J.; Chen, E.C. Synthesis and mesomorphic behaviour of the series of 2-(4-alkoxyphenyl)-6-methoxyquinolines and 2-(4-alkoxybiphenyl-4'-yl)-6-methoxyquinoline. Liq. Cryst. 2013, 40, 989–997. [Google Scholar] [CrossRef]
- Chia, W.L.; Cheng, Y.W. Facile synthesis of a series of 2-(4-alkyloxyphenyl)-5-cyanopyridine liquid crystalline compounds. Heterocycles 2008, 75, 375–382. [Google Scholar] [CrossRef]
- Chia, W.L.; Li, C.L.; Lin, C.H. Synthesis and mesomorphic studies on the series of 2-(4-alkoxyphenyl)-5-phenylpyridines and 2-(6-alkoxynaphthalen-2-yl)-5-phenylpyridines. Liq. Cryst. 2010, 37, 23–30. [Google Scholar] [CrossRef]
- Chia, W.L.; Tsai, C.Y. Synthesis and mesomorphic properties of a series of phenyl 6-(4-alkoxyphenyl)nicotinates. Heterocycles 2011, 83, 1057–1065. [Google Scholar] [CrossRef]
- Chia, W.L.; Lin, C.W. Synthesis and thermotropic studies of a novel series of nematogenic liquid crystals 2-(6-alkoxynaphthalen-2-yl)-5-cyanopyridines. Liq. Cryst. 2013, 40, 922–931. [Google Scholar] [CrossRef]
- Comins, D.L.; Abdullah, A.H. Regioselective addition of Grignard reagents to 1-acylpyridinium salts. A convenient method for the synthesis of 4-alkyl(aryl)pyridines. J. Org. Chem. 1982, 47, 4315–4319. [Google Scholar] [CrossRef]
- Comins, D.L.; Stroud, E.D.; Herrick, J.J. Regioselective addition of Grignard reagents to the 1-phenoxycarbonyl salts of alkyl nicotinates. Heterocycles 1984, 22, 151–157. [Google Scholar] [CrossRef]
- Dierking, I. Polarizing microscopy. In Textures of Liquid Crystals; WILEY-VCH Verlag: Weinheim, Germany, 2003; pp. 33–42. [Google Scholar]
- Gray, G.W.; Mosley, A. Trends in the nematic-isotropic liquid transition temperatures for the homologous series of 4-n-alkoxy and 4-n-alkyl-4' cyanobiphenyls. J. Chem. Soc. Perkin II 1976, 2, 97–102. [Google Scholar] [CrossRef]
- Imrie, C.T.; Taylor, L. The preparation and properties of low molar mass liquid-crystals possessing lateral alkyl chains. Liq. Cryst. 1989, 6, 1–10. [Google Scholar] [CrossRef]
- Attard, G.S.; Imrie, C.T. Liquid-crystalline and glass-forming dimers derived from 1-aminopyrene. Liq. Cryst. 1992, 11, 785–789. [Google Scholar] [CrossRef]
- Donaldson, T.; Staesche, H.; Lu, Z.B.; Henderson, P.A.; Achard, M.F.; Imrie, C.T. Symmetric and non-symmetric chiral liquid crystal dimers. Liq. Cryst. 2010, 37, 1097–1110. [Google Scholar] [CrossRef]
- Chan, T.-N.; Lu, Z.B.; Yam, W.-S.; Yeap, G.Y.; Imrie, C.T. Non-symmetric liquid crystal dimers containing an isoflavone moiety. Liq. Cryst. 2012, 39, 393–402. [Google Scholar] [CrossRef]
- Asano, T.; Uenoyama, M.; Moriya, K.; Yano, S.; Takatani, S.; Kagabu, S. Polymorphism in a homologous series of 2-(4-alkoxyphenyl)-5-(4-methylphenyl)pyridines. Liq. Cryst. 1997, 23, 365–369. [Google Scholar] [CrossRef]
- Zang, Z.Q.; Zhang, D.D.; Wan, X.H.; Zhou, Q.F. The synthesis and property of liquid crystalline 4-alkoxyl-4"-cyano-p-terphenyls. Mol. Cryst. Liq. Cryst. 2000, 339, 145–158. [Google Scholar] [CrossRef]
- Chia, W.-L.; Kuo, K.-N.; Lin, S.-H. Synthesis and thermotropic studies of two novel series of kinked liquid crystals: 2-(4'-alkoxybiphen-4-yl)-6-methylquinolines and 2-(6'-alkoxynaphthalen-2-yl)-6-methylquinolines. Int. J. Mol. Sci. 2014, 15, 7579–7593. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chia, W.-L.; Liu, C.-W. A New Series of Kinked Liquid Crystals: 2-(6-Alkoxynaphthalen-2-yl)-6-methoxyquinolines. Int. J. Mol. Sci. 2015, 16, 7520-7534. https://doi.org/10.3390/ijms16047520
Chia W-L, Liu C-W. A New Series of Kinked Liquid Crystals: 2-(6-Alkoxynaphthalen-2-yl)-6-methoxyquinolines. International Journal of Molecular Sciences. 2015; 16(4):7520-7534. https://doi.org/10.3390/ijms16047520
Chicago/Turabian StyleChia, Win-Long, and Chih-Wei Liu. 2015. "A New Series of Kinked Liquid Crystals: 2-(6-Alkoxynaphthalen-2-yl)-6-methoxyquinolines" International Journal of Molecular Sciences 16, no. 4: 7520-7534. https://doi.org/10.3390/ijms16047520
APA StyleChia, W. -L., & Liu, C. -W. (2015). A New Series of Kinked Liquid Crystals: 2-(6-Alkoxynaphthalen-2-yl)-6-methoxyquinolines. International Journal of Molecular Sciences, 16(4), 7520-7534. https://doi.org/10.3390/ijms16047520