Posttraumatic Inflammation as a Key to Neuroregeneration after Traumatic Spinal Cord Injury
Abstract
:1. Introduction
- (1)
- Is it possible to quantitatively measure the factors in the serum of traumatic SCI patients according to neurological impairment?
- (2)
- Do the serum levels of patients with different neurological outcome differ from one another?
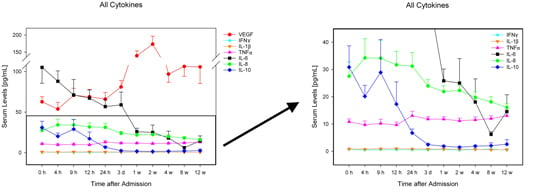
2. Results
2.1. Patient Demographics
| Patients | N | Age (years) | Gender | Etiology | AO | NLI | Initial AIS | Final AIS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | Fall | Traffic | Other | A | B | C | C | Th | L | A | B | C | D | A | B | C | D | |||
| All Patients | 40 | 43.55 ± 20.80 | 12 | 28 | 25 | 13 | 2 | 19 | 13 | 5 | 17 | 11 | 12 | 20 | 6 | 13 | 1 | 14 | 4 | 7 | 15 |
| Remission (G1) | 23 | 43.26 ± 23.56 | 10 | 13 | 15 | 7 | 1 | 12 | 7 | 2 | 8 | 6 | 9 | 6 | 5 | 12 | 0 | 0 | 3 | 6 | 14 |
| No Remission (G2) | 17 | 43.94 ± 17.05 | 2 | 15 | 10 | 6 | 1 | 7 | 6 | 3 | 9 | 5 | 3 | 14 | 1 | 1 | 1 | 14 | 1 | 1 | 1 |
| p > 0.05 | p < 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p < 0.05 | p < 0.05 | |||||||||||||||
2.2. Analysis of Entire Patient Collective





2.3. Comparison of Patients with Neurological Remission vs. without Neurological Remission (G1 vs. G2)
2.4. Comparison of Patients with AIS A and AIS B–D
2.4.1. AIS A ↔ and AIS A ↑
2.4.2. AIS B–D ↔ and AIS B–D ↑
2.4.3. AIS A and AIS B–D
2.4.4. Receiver Operating Characteristic (ROC) Analysis
| Factor | Time after Admission | |
|---|---|---|
| h0 | h9 | |
| IL-8 | 22.41 pg/mL | |
| Sensitivity | 0.813 | |
| Specificity | 0.842 | |
| Area Under the Curve | 0.852 * | |
| IL-10 | 4.83 pg/mL | |
| Sensitivity | 0.923 | |
| Specificity | 0.692 | |
| Area Under the Curve | 0.831 † | |
2.5. Data Analysis
3. Material and Methods
| AIS Grade | Clinical State |
|---|---|
| A | Complete—No motor or sensory function is preserved in the sacral segments S4–S5 |
| B | Incomplete—Sensory but not motor function is preserved below the NLI and includes the sacral segments S4–S5 |
| C | Incomplete—Motor function is preserved below the NLI, and more than half of key muscles below the NLI have a muscle grade less than 3 |
| D | Incomplete—Motor function is preserved below the NLI, and at least half of key muscles below the NLI have a muscle grade of 3 or more |
| E | Normal—Motor and sensory function is normal |
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Devivo, M.J. Epidemiology of traumatic spinal cord injury: Trends and future implications. Spinal Cord 2012, 50, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Boakye, M.; Leigh, B.C.; Skelly, A.C. Quality of life in persons with spinal cord injury: Comparisons with other populations. J. Neurosurg. Spine 2012, 17, 29–37. [Google Scholar] [PubMed]
- DeVivo, M.J. Causes and costs of spinal cord injury in the United States. Spinal Cord 1997, 35, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.K.; Stammers, A.M.; Belanger, L.M.; Bernardo, A.; Chan, D.; Bishop, C.M.; Slobogean, G.P.; Zhang, H.; Umedaly, H.; Giffin, M.; et al. Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J. Neurotrauma 2010, 27, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D.; Smith, J.; Marcillo, A. The pathology of human spinal cord injury: Defining the problems. J. Neurotrauma 2004, 21, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H.; Koyanagi, I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J. Neurosurg. 1997, 86, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Keane, R.W.; Davis, A.R.; Dietrich, W.D. Inflammatory and apoptotic signaling after spinal cord injury. J. Neurotrauma 2006, 23, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Baptiste, D.C. Current status of clinical trials for acute spinal cord injury. Injury 2005, 36, B113–B122. [Google Scholar] [CrossRef] [PubMed]
- Stammers, A.T.; Liu, J.; Kwon, B.K. Expression of inflammatory cytokines following acute spinal cord injury in a rodent model. J. Neurosci. Res. 2012, 90, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Ueyama, T.; Nemoto, K.; Tamaki, T.; Senba, E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J. Neurotrauma 2000, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Klusman, I.; Schwab, M.E. Effects of pro-inflammatory cytokines in experimental spinal cord injury. Brain Res. 1997, 762, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Thangnipon, W.; McAdoo, D.J. Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res. 1991, 547, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Biglari, B.; Buchler, A.; Swing, T.; Biehl, E.; Roth, H.J.; Bruckner, T.; Schmidmaier, G.; Ferbert, T.; Gerner, H.J.; Moghaddam, A. Increase in soluble CD95L during subacute phases after human spinal cord injury: A potential therapeutic target. Spinal Cord 2013, 51, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Biglari, B.; Buchler, A.; Swing, T.; Child, C.; Biehl, E.; Reitzel, T.; Bruckner, T.; Ferbert, T.; Korff, S.; Rief, H.; et al. Serum sCD95L concentration in patients with spinal cord injury. J. Int. Med. Res. 2015, in press. [Google Scholar]
- Biglari, B.; Swing, T.; Child, C.; Büchler, A.; Westhauser, F.; Bruckner, T.; Ferbert, T.; Gerner, H.; Moghaddam, A. A pilot study on temporal changes in IL-1β and TNF-α serum levels after spinal cord injury: The serum level of TNF-α in acute SCI patients as a possible marker for neurological remission. J. Spinal Cord Med. 2015, in press. [Google Scholar]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.J.; Ditunno, J.F., Jr.; Donovan, W.H.; Maynard, F., Jr. Neurologic recovery after traumatic spinal cord injury: Data from the Model Spinal Cord Injury Systems. Arch. Phys. Med. Rehabil. 1999, 80, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.L.; Hayes, K.C.; Dekaban, G.A. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 2007, 88, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.C.; Hull, T.C.; Delaney, G.A.; Potter, P.J.; Sequeira, K.A.; Campbell, K.; Popovich, P.G. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J. Neurotrauma 2002, 19, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.R.; Welsh, C.J.; Young, C.; Spoor, E.; Kerwin, S.C.; Griffin, J.F.; Levine, G.J.; Cohen, N.D.; Levine, J.M. Cerebrospinal fluid inflammatory cytokines and chemokines in naturally occurring canine spinal cord injury. J. Neurotrauma 2014, 31, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Urdzikova, L.M.; Ruzicka, J.; LaBagnara, M.; Karova, K.; Kubinova, S.; Jirakova, K.; Murali, R.; Sykova, E.; Jhanwar-Uniyal, M.; Jendelova, P. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int. J. Mol. Sci. 2014, 15, 11275–11293. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Yoles, E. Immune-based therapy for spinal cord repair: Autologous macrophages and beyond. J. Neurotrauma 2006, 23, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Pineau, I.; Sun, L.; Bastien, D.; Lacroix, S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav. Immun. 2010, 24, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.H.; Tu, W.Z.; Zou, E.M.; Hu, J.; Wang, S.; Li, J.R.; Wang, W.S.; He, R.; Cheng, R.D.; Liao, W.J. Neuroprotective effects of different modalities of acupuncture on traumatic spinal cord injury in rats. Evid.-Based Complement. Altern. Med. eCAM 2014, 2014, 431580. [Google Scholar]
- Hou, Y.; Ryu, C.H.; Jun, J.A.; Kim, S.M.; Jeong, C.H.; Jeun, S.S. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol. Int. 2014, 38, 1050–1059. [Google Scholar] [PubMed]
- Tsai, M.C.; Wei, C.P.; Lee, D.Y.; Tseng, Y.T.; Tsai, M.D.; Shih, Y.L.; Lee, Y.H.; Chang, S.F.; Leu, S.J. Inflammatory mediators of cerebrospinal fluid from patients with spinal cord injury. Surg. Neurol. 2008, 70, 19–24. [Google Scholar] [CrossRef]
- Ishii, H.; Tanabe, S.; Ueno, M.; Kubo, T.; Kayama, H.; Serada, S.; Fujimoto, M.; Takeda, K.; Naka, T.; Yamashita, T. IFN-γ-dependent secretion of IL-10 from Th1 cells and microglia/macrophages contributes to functional recovery after spinal cord injury. Cell Death Dis. 2013, 4, e710. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Han, M.; Zhou, J.; Zheng, Z.F.; Lu, P.; Wang, J.J.; Wang, J.Q.; Mao, Q.J.; Gao, J.Q.; Ouyang, H.W. Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials 2014, 35, 6585–6594. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.H.; Lim, J.E.; Chi, G.F.; Ahn, W.; Zhang, M.; Chung, E.; Son, Y. Substance P reduces apoptotic cell death possibly by modulating the immune response at the early stage after spinal cord injury. Neuroreport 2013, 24, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Ohtaki, H.; Tsumuraya, T.; Song, D.; Ohara, K.; Asano, M.; Iwakura, Y.; Atsumi, T.; Shioda, S. Interleukin-1 participates in the classical and alternative activation of microglia/macrophages after spinal cord injury. J. Neuroinflamm. 2012, 9, 65. [Google Scholar] [CrossRef]
- Vidal, P.M.; Lemmens, E.; Geboes, L.; Vangansewinkel, T.; Nelissen, S.; Hendrix, S. Late blocking of peripheral TNF-α is ineffective after spinal cord injury in mice. Immunobiology 2013, 218, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Garraway, S.M.; Woller, S.A.; Huie, J.R.; Hartman, J.J.; Hook, M.A.; Miranda, R.C.; Huang, Y.J.; Ferguson, A.R.; Grau, J.W. Peripheral noxious stimulation reduces withdrawal threshold to mechanical stimuli after spinal cord injury: Role of tumor necrosis factor α and apoptosis. Pain 2014, 155, 2344–2359. [Google Scholar] [CrossRef] [PubMed]
- Hook, M.A.; Washburn, S.N.; Moreno, G.; Woller, S.A.; Puga, D.; Lee, K.H.; Grau, J.W. An IL-1 receptor antagonist blocks a morphine-induced attenuation of locomotor recovery after spinal cord injury. Brain Behav. Immun. 2011, 25, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Pereira, F.C.; Marcillo, A.E.; Bates, M.L.; Berrocal, Y.A.; Filbin, M.T.; Bunge, M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004, 10, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Zimmermann, G.; Pufe, T.; Varoga, D.; Henle, P. The systemic angiogenic response during bone healing. Arch. Orthop. Trauma Surg. 2009, 129, 989–997. [Google Scholar] [CrossRef] [PubMed]
- LaPar, D.J.; Rosenberger, L.H.; Walters, D.M.; Hedrick, T.L.; Swenson, B.R.; Young, J.S.; Dossett, L.A.; May, A.K.; Sawyer, R.G. Severe traumatic head injury affects systemic cytokine expression. J. Am. Coll. Surg. 2012, 214, 478–486. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghaddam, A.; Child, C.; Bruckner, T.; Gerner, H.J.; Daniel, V.; Biglari, B. Posttraumatic Inflammation as a Key to Neuroregeneration after Traumatic Spinal Cord Injury. Int. J. Mol. Sci. 2015, 16, 7900-7916. https://doi.org/10.3390/ijms16047900
Moghaddam A, Child C, Bruckner T, Gerner HJ, Daniel V, Biglari B. Posttraumatic Inflammation as a Key to Neuroregeneration after Traumatic Spinal Cord Injury. International Journal of Molecular Sciences. 2015; 16(4):7900-7916. https://doi.org/10.3390/ijms16047900
Chicago/Turabian StyleMoghaddam, Arash, Christopher Child, Thomas Bruckner, Hans Jürgen Gerner, Volker Daniel, and Bahram Biglari. 2015. "Posttraumatic Inflammation as a Key to Neuroregeneration after Traumatic Spinal Cord Injury" International Journal of Molecular Sciences 16, no. 4: 7900-7916. https://doi.org/10.3390/ijms16047900
APA StyleMoghaddam, A., Child, C., Bruckner, T., Gerner, H. J., Daniel, V., & Biglari, B. (2015). Posttraumatic Inflammation as a Key to Neuroregeneration after Traumatic Spinal Cord Injury. International Journal of Molecular Sciences, 16(4), 7900-7916. https://doi.org/10.3390/ijms16047900