Antimicrobial Action of Water-Soluble β-Chitosan against Clinical Multi-Drug Resistant Bacteria
Abstract
:1. Introduction
2. Results and Discussion
2.1. A Potent Antibacterial Activity of LMWS β-Chitosan
| Bacteria | Minimum Inhibitory Concentration (MIC, mg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α1k | α5k | α10k | β1k | β5k | β10k | |||||||
| pH | 7.4 | 5.4 | 7.4 | 5.4 | 7.4 | 5.4 | 7.4 | 5.4 | 7.4 | 5.4 | 7.4 | 5.4 |
| Gram(−) bacteria | ||||||||||||
| E. coli | >5 | 0.156 | >5 | 0.039 | 0.009 | 0.009 | 0.625 | 1.25 | 0.018 | 0.009 | 0.009 | 0.009 |
| E. coli O157 | >5 | 0.009 | >5 | 0.009 | 0.009 | 0.009 | 0.625 | 1.25 | 0.009 | 0.009 | 0.009 | 0.009 |
| P. aeruginosa | >5 | 0.156 | >5 | 0.078 | 0.009 | 0.009 | 0.625 | 1.25 | 0.009 | 0.009 | 0.009 | 0.009 |
| S. typhimurium | >5 | 0.045 | >5 | 0.009 | 0.009 | 0.005 | 0.313 | 1.25 | 0.018 | 0.009 | 0.009 | 0.009 |
| L. monocytogens | >5 | 0.313 | >5 | 0.156 | 0.009 | 0.009 | 0.313 | 2.5 | 0.009 | 0.009 | 0.009 | 0.009 |
| Gram(+) bacteria | ||||||||||||
| B. cereus | >5 | 0.018 | >5 | 0.018 | 4.5 | 0.009 | 0.625 | 0.625 | 0.009 | 0.009 | 0.009 | 0.009 |
| B. megaterium | 2500 | 0.018 | 313 | 0.018 | 0.009 | 0.009 | 0.313 | 1.25 | 0.009 | 0.009 | 0.009 | 0.009 |
| S. aureus | >5 | 0.039 | >5 | 0.018 | 0.009 | 0.009 | 1.25 | 2.5 | 0.018 | 0.009 | 0.009 | 0.009 |
| S. epidermidis | 5 | 0.009 | 5 | 0.045 | 0.018 | 0.005 | 0.625 | 2.5 | 0.018 | 0.009 | 0.018 | 0.005 |
| Bacteria | MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| β5k | β10k | PIP a | AMP a | CIP a | GM a | TET a | |
| Gram(−) bacteria | |||||||
| P. aeruginosa ATCC 27853 | 9 | 9 | <4 | <2 | 0.25 | 0.5 | – |
| P. aeruginosa BMP-Pa001 | <4.5 | <4.5 | >128 | >32 | 2 | 1 | – |
| P. aeruginosa BMP-Pa002 | <4.5 | <4.5 | 16 | >32 | >8 | 8 | – |
| P. aeruginosa BMP-Pa003 | <4.5 | <4.5 | >128 | >32 | >8 | 32 | – |
| P. aeruginosa BMP-Pa004 | <4.5 | <4.5 | 16 | >32 | 0.25 | 1 | – |
| P. aeruginosa BMP-Pa005 | <4.5 | <4.5 | >128 | >32 | 0.25 | 8 | – |
| Gram(+) bacteria | |||||||
| S. aureus ATCC 25923 | 18 | 9 | – | – | 0.25 | 0.25 | 0.5 |
| S. aureus BMP-Sa001 | <4.5 | <4.5 | – | – | 1 | 0.25 | >16 |
| S. aureus BMP-Sa002 | 39 | 18 | – | – | >8 | >16 | 1 |
| S. aureus BMP-Sa003 | 39 | 18 | – | – | >8 | >16 | >16 |
| S. aureus BMP-Sa004 | 18 | 9 | – | – | >8 | >16 | >16 |
| S. aureus BMP-Sa005 | 39 | 18 | – | – | >8 | >16 | >16 |
2.2. Cytotoxic Effect of Chitosan

2.3. Membranolytic Action of β-Chitosan



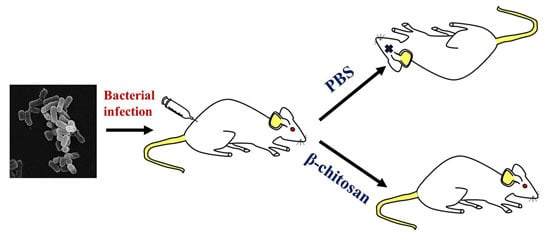
2.4. In Vivo Antibacterial Effect of β-Chitosan


3. Experimental Section
3.1. Materials
3.2. Preparation of Low Molecular Weight Water-Soluble Chitosans (LMWSC)
3.3. Bacterial Strains
3.4. Antibacterial Activity
3.5. Kinetics of Bacterial Killing
3.6. Hemolysis
3.7. Rhodamine Release from Liposomes
3.8. Morphological Observation by Scanning Electron Microscopy
3.9. In Vivo Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Molton, J.S.; Tambyah, P.A.; Ang, B.S.; Ling, M.L.; Fisher, D.A. The global spread of healthcare-associated multidrug-resistant bacteria: A perspective from Asia. Clin. Infect. Dis. 2013, 56, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Park, Y.; Hahm, K.S. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Dey, D.; Hazra, S.; Ghosh, S.; Ray, R.; Hazra, B. Antibacterial and antifungal activity of Terminalia arjuna wight & Arn bark against multi-drug resistant clinical isolates. J. Coast. Life Med. 2013, 1, 312–318. [Google Scholar]
- Nicole, A.J.; Amy, Y.T.Y.; Olga, M.P.; Robert, E.W.H. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Hayama, K.; Ishibashi, H.; Ishijima, S.A.; Niimi, K.; Tansho, S.; Ono, Y.; Monk, B.C.; Holmes, A.R.; Harding, D.R.K.; Cannon, R.D.; et al. A d-octapeptide drug efflux pump inhibitor acts synergistically with azoles in a murine oral candidiasis infection model. FEMS Microbiol. Lett. 2012, 328, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Pagès, J.M.; Amaral, L.; Fanning, S. An original deal for new molecule: Reversal of efflux pump activity, a rational strategy to combat gram-negative resistant bacteria. Curr. Med. Chem. 2011, 18, 2969–2980. [Google Scholar] [CrossRef] [PubMed]
- Schindler, B.D.; Jacinto, P.; Kaatz, G.W. Inhibition of drug efflux pumps in Staphylococcus aureus: Current status of potentiating existing antibiotics. Future Microbiol. 2013, 8, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2013, 43, 145–171. [Google Scholar] [CrossRef]
- Sikorski, P.; Hori, R.; Wada, M. Revisit of α-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 2009, 10, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Sawada, D.; Kimura, S.; Nishiyama, Y.; Langan, P.; Wada, M. The crystal structure of mono-ethylenediamine β-chitin from synchrotron X-ray fiber diffraction. Carbohydr. Polym. 2013, 92, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.C.; Luke, B.M. A two-system model for chitin-protein complexes in insect cuticles. Tissue Cell 1969, 1, 689–707. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ahmad, S.R. Polysaccharides and their derivatives for versatile tissue engineering application. Macromol. Biosci. 2013, 13, 395–421. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Juneja, V.K. Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [PubMed]
- Hayes, M.; Carney, B.; Slater, J.; Brück, W. Mining marine shellfish wastes for bioactive molecules: Chitin and chitosan—Part B: Applications. Biotechnol. J. 2008, 3, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Firdous, J.; Choi, Y.J.; Yun, C.H.; Cho, C.S. Design and application of chitosan microspheres as oral and nasal vaccine carriers: An updated review. Int. J. Nanomed. 2012, 7, 6077–6093. [Google Scholar]
- Park, S.C.; Nah, J.W.; Park, Y. pH-dependent mode of antibacterial actions of low molecular weight water-soluble chitosan (LMWSC) against various pathogens. Macromol. Res. 2011, 19, 853–860. [Google Scholar] [CrossRef]
- Pontoni, L.; Fabbricino, M. Use of chitosan and chitosan-derivatives to remove arsenic from aqueous solutions—A mini review. Carbohydr. Res. 2012, 356, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Zhao, Y. Impact of the structural differences between α- and β-chitosan on their depolymerizing reaction and antibacterial activity. J. Agric. Food Chem. 2013, 61, 8783–8789. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Zhao, Y. Effect of molecular weight, acid, and plasticizer on the physicochemical and antibacterial properties of β-chitosan based films. J. Food Sci. 2012, 77, E127–136. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, Y.; Zhao, Y. Physicochemical, microstructural, and antibacterial properties of β-chitosan and kudzu starch composite films. J. Food Sci. 2012, 77, E280–E286. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kimura, S.; Wada, M. Electron diffraction and high-resolution imaging on highly-crystalline β-chitin microfibril. J. Struct. Biol. 2011, 176, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sawada, D.; Nishiyama, Y.; Langan, P.; Forsyth, V.T.; Kimura, S.; Wada, M. Water in crystalline fibers of dihydrate β-chitin results in unexpected absence of intramolecular hydrogen bonding. PLoS ONE 2012, 7, e39376. [Google Scholar] [CrossRef] [PubMed]
- Sawada, D.; Nishiyama, Y.; Langan, P.; Forsyth, V.T.; Kimura, S.; Wada, M. Direct determination of the hydrogen bonding arrangement in anhydrous β-chitin by neutron fiber diffraction. Biomacromolecules 2012, 9, 288–291. [Google Scholar] [CrossRef]
- Nah, J.W.; Jang, M.K. Spectroscopic characterization and preparation of low molecular, water-soluble chitosan with free-amine group by novel method. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3796–3803. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-C.; Nam, J.-P.; Kim, J.-H.; Kim, Y.-M.; Nah, J.-W.; Jang, M.-K. Antimicrobial Action of Water-Soluble β-Chitosan against Clinical Multi-Drug Resistant Bacteria. Int. J. Mol. Sci. 2015, 16, 7995-8007. https://doi.org/10.3390/ijms16047995
Park S-C, Nam J-P, Kim J-H, Kim Y-M, Nah J-W, Jang M-K. Antimicrobial Action of Water-Soluble β-Chitosan against Clinical Multi-Drug Resistant Bacteria. International Journal of Molecular Sciences. 2015; 16(4):7995-8007. https://doi.org/10.3390/ijms16047995
Chicago/Turabian StylePark, Seong-Cheol, Joung-Pyo Nam, Jun-Ho Kim, Young-Min Kim, Jae-Woon Nah, and Mi-Kyeong Jang. 2015. "Antimicrobial Action of Water-Soluble β-Chitosan against Clinical Multi-Drug Resistant Bacteria" International Journal of Molecular Sciences 16, no. 4: 7995-8007. https://doi.org/10.3390/ijms16047995
APA StylePark, S. -C., Nam, J. -P., Kim, J. -H., Kim, Y. -M., Nah, J. -W., & Jang, M. -K. (2015). Antimicrobial Action of Water-Soluble β-Chitosan against Clinical Multi-Drug Resistant Bacteria. International Journal of Molecular Sciences, 16(4), 7995-8007. https://doi.org/10.3390/ijms16047995