Reducing the Oxidation Level of Dextran Aldehyde in a Chitosan/Dextran-Based Surgical Hydrogel Increases Biocompatibility and Decreases Antimicrobial Efficacy
Abstract
:1. Introduction
2. Results
2.1. Minimum Inhibitory and Minimum Bactericidal Determinations
| Microorganism | American Type Culture Collection Number | Antibiotic Control |
|---|---|---|
| Staphylococcus aureus | ATCC 9144 | Penicillin G |
| Escherichia coli | ATCC 25922 | Gentamicin |
| Enterococcus faecalis | ATCC 29212 | Gentamicin + Penicillin G |
| Bacteroides fragilis | ATCC 25285 | Metronidazole |
| Peptostreptococcus anaerobius | ATCC 27337 | Metronidazole |
| Clostridium perfringens | ATCC 13124 | Metronidazole |
| Helicobacter pylori | ATCC 11637 | Metronidazole |
| Organism | Concentration (mg·mL−1) | Positive Controls a (μg·mL−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DA-25 | CD-25 | DA-100 | CD-100 | |||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Escherichia coli | >64 | >64 | >50 | >50 | 32 | 50 | >50 | >50 | 8 | 8 |
| Staphylococcus aureus | >64 | >64 | >50 | >50 | 8 | 32 | 40 | 40 | 0.0625 | 1 |
| Enterocococcus faecalis | >64 | >64 | >50 | >50 | 8 | 25 | >50 | >50 | 0.5 | 1 |
| Peptostreptococcus anaerobius | 50 | 50 | >50 | >50 | 4 | 8 | 40 | 40 | 0.5 | 1 |
| Bacteroides fragilis | 50 | 50 | >50 | >50 | 4 | 8 | 20 | 40 | 0.5 | 1 |
| Clostridium perfringens | 32 | 32 | >50 | >50 | 2 | 2 | 20 | 20 | 1 | 1 |
| Helicobacter pylori | 8 | 8 | >50 | >50 | 2 | 2 | 20 | 20 | 1 | 1 |
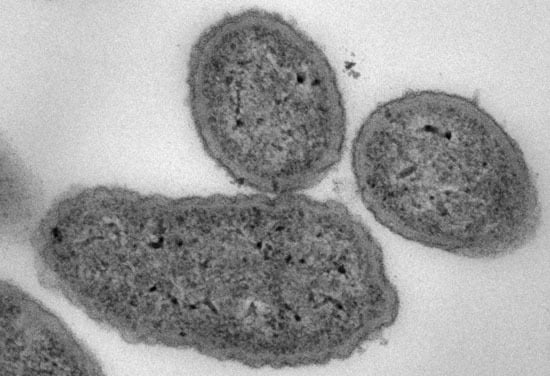
2.2. Antimicrobial Mode of Action


2.3. Cytotoxicity Assay

2.4. In Vitro Wound Healing Assay

3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bacterial Strains
4.3. Preparation of DA, SC, and CD Hydrogel
4.4. Antimicrobial Efficacy Testing
| CD hydrogel Final Concentration (mg·mL−1) | Concentration of CD Hydrogel Components (mg·mL−1) | |
|---|---|---|
| Succinyl Chitosan (SC) | Dextran Aldehyde (DA) | |
| 50 | 50 | 50 |
| 40 a | 50 | 30 |
| 25 b | 40 | 10 |
| 20 | 25 | 15 |
| 10 | 12.5 | 7.5 |
| 5 | 6.25 | 3.75 |
| 2.5 | 3.125 | 1.875 |
| 1.25 | 1.56 | 0.94 |
| Compounds | Concentration (mg·mL−1) |
|---|---|
| DA-100 | 64, 50, 32, 16, 8, 4, 2 |
| DA-25 | 64, 50, 32, 16, 8, 4, 2 |
| CD-100 | 50, 40, 25, 20, 10, 5, 2.5, 1.25 |
| CD-25 | 50, 40, 25, 20, 10, 5, 2.5, 1.25 |
| a Penicillin G | 0.032, 0.016, 0.008, 0.004, 0.002, 0.001, 0.0005, 0.00025, 0.000125 |
| a Gentamicin | 0.032, 0.016, 0.008, 0.004, 0.002, 0.001, 0.0005, 0.00025 |
| a Metronidazole | 0.016, 0.008, 0.004, 0.002, 0.001, 0.0005, 0.00025, 0.000125 |
| a Penicillin G + Gentamicin | 0.032/0.032, 0.016/0.016, 0.008/0.008, 0.004/0.004, 0.002/0.002, 0.001/0.001, 0.0005/0.0005 |
4.5. Transmission Electron Microscopy (TEM)
4.6. Cytotoxicity Assay
4.7. In Vitro Wound Healing Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peppas, N.A.; Huang, Y.; Torres-Lugo, M.; Ward, J.H.; Zhang, J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu. Rev. Biomed. Eng. 2000, 2, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.D.; Roxburgh, M.; Shi, Z.; Liu, L.; McConnell, M.; Williams, G.; Evans, N.; Hanton, L.R.; Simpson, J.; Moratti, S.C.; et al. Synthesis, physiochemical characterization, and biocompatibility of a chitosan/dextran-based hydrogel for postsurgical adhesion prevention. J. Mater. Sci. Mater. Med. 2014, 25, 2743–2756. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.E.; Cohen, Z.; Fleshman, J.W.; Kaufman, H.S.; van Goor, H.; Wolff, B.G. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm® adhesion barrier in abdominopelvic surgery of the intestine. Dis. Colon Rectum 2003, 46, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Fazio, V.W.; Cohen, Z.; Fleshman, J.W.; van Goor, H.; Bauer, J.J.; Wolff, B.G.; Corman, M.; Beart, R.W., Jr.; Wexner, S.D.; Becker, J.M.; et al. Reduction in adhesive small-bowel obstruction by Seprafilm® adhesion barrier after intestinal resection. Dis. Colon Rectum 2006, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Haney, A.F.; Doty, E. Murine peritoneal injury and de novo adhesion formation caused by oxidized-regenerated cellulose (Interceed [TC7]) but not expanded polytetrafluoroethylene (Gore-Tex Surgical Membrane). Fertil. Steril. 1992, 57, 202–208. [Google Scholar] [PubMed]
- Wiseman, D.M.; Gottlick-Iarkowski, L.; Kamp, L. Effect of different barriers of oxidized regenerated cellulose (ORC) on cecal and sidewall adhesions in the presence and absence of bleeding. Investig. Surg. 1999, 12, 141–146. [Google Scholar]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Cabral, J.D.; Brooks, H.J.; Moratti, S.C.; Hanton, L.R. Antimicrobial properties of a chitosan dextran-based hydrogel for surgical use. Antimicrob. Agents Chemother. 2012, 56, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Vedyashkina, T.A.; Revin, V.V.; Gogotov, I.N. Optimizing the conditions of dextran synthesis by the bacterium Leuconostoc mesenteroides grown in a molasses-containing medium. Appl. Biochem. Microbiol. 2005, 41, 361–364. [Google Scholar] [CrossRef]
- Kulicke, W.M.; Heinze, T. Improvements in polysaccharides for use as blood plasma expanders. Macromol. Symp. 2006, 231, 47–59. [Google Scholar] [CrossRef]
- Matthews, W.B.; Oxbury, J.M.; Grainger, K.M.; Greenhall, R.C. A blind controlled trial of dextran 40 in the treatment of ischaemic stroke. Brain J. Neurol. 1976, 99, 193–206. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Athanasiadis, T.; Beule, A.G.; Robinson, B.H.; Robinson, S.R.; Shi, Z.; Wormald, P.J. Effects of a novel chitosan gel on mucosal wound healing following endoscopic sinus surgery in a sheep model of chronic rhinosinusitis. Laryngoscope 2008, 118, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.; Athanasiadis, T.; Moratti, S.; Hanton, L.; Robinson, S.; Wormald, P.J. The efficacy of a novel chitosan gel on hemostasis and wound healing after endoscopic sinus surgery. Am. J. Rhinol. Allergy 2010, 24, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lauder, C.I.; Garcea, G.; Strickland, A.; Maddern, G.J. Use of a modified chitosan-dextran gel to prevent peritoneal adhesions in a rat model. J. Surg. Res. 2011, 171, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Cabral, J.D.; Brooks, H.J.; McConnell, M.A.; Fitzpatrick, C.; Hanton, L.R.; Moratti, S.C. In vitro biocompatibility and cellular interactions of a chitosan/dextran-based hydrogel for postsurgical adhesion prevention. J. Biomed. Mater. Res. Part B 2015, 103, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Moscowitz, I.; Wexner, S.D. Contributions of adhesions to the cost of healthcare. Perit. Surg. 2000, 335–342. [Google Scholar]
- Okabayashi, K.; Ashrafian, H.; Zacharakis, E.; Hasegawa, H.; Kitagawa, Y.; Athanasiou, T.; Darzi, A. Adhesions after abdominal surgery: A systematic review of the incidence, distribution and severity. Surg. Today 2014, 44, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Hospital Infection Control Practices Advisory Committee; Mangaram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for prevention of surgical site infection. Infect. Control Hosp. Epidemiol. 1999, 20, 279–280. [Google Scholar]
- Montravers, P.; Lepape, A.; Dubreuil, L.; Gauzit, R.; Pean, Y.; Benchimol, D.; Dupont, H. Clinical and microbiological profiles of community-acquired and nosocomial intra-abdominal infections: Results of the French prospective, observational EBIIA study. J. Antimicrob. Chemother. 2009, 63, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Lauder, C.I.; Strickland, A.; Maddern, G.J. Use of a modified chitosan-dextran gel to prevent peritoneal adhesions in a porcine hemicolectomy model. J. Surg. Res. 2012, 176, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Rajiv, S.; Harding, M.; Bassiouni, A.; Jardeleza, C.; Drilling, A.; James, C.; Ha, T.; Moratti, S.; Robinson, S.; Wormald, P.J. The efficacy and safety of chitosan dextran gel in a burr hole neurosurgical sheep model. Acta Neurochir. 2013, 155, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Deng, X.; Wei, X.; Shi, H.; Wang, F.; Ye, T.; Shao, B.; Nie, W.; Li, Y.; Luo, M.; et al. Novel thermosensitive hydrogel for preventing formation of abdominal adhesions. Int. J. Nanomed. 2012, 8, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.C.; Panitch, A. Abdominal adhesions: Current and novel therapies. J. Surg. Res. 2011, 165, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.; Athanasiadis, T.; Moratti, S.; Robinson, S.; Wormald, P.J. The efficacy of a novel chitosan gel on hemostasis after endoscopic sinus surgery in a sheep model of chronic rhinosinusitis. Am. J. Rhinol. Allergy 2009, 23, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Giano, M.C.; Ibrahim, Z.; Medina, S.H.; Sarhane, K.A.; Christensen, J.M.; Yamada, Y.; Brandacher, G.; Schneider, J.P. Injectable bioadhesive hydrogels with innate antibacterial properties. Nat. Commun. 2014, 5, 4905. [Google Scholar] [CrossRef] [PubMed]
- Bruck, C. Role of glutaraldehyde and other liquid chemical sterilants in the processing of new medical devices. Steriliz. Med. Prod. 1991, 5, 376–396. [Google Scholar]
- Hughes, R.C.; Thurman, P.F. Cross-linking of bacterial cell walls with glutaraldehyde. Biochem. J. 1970, 119, 925. [Google Scholar] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Worth, L.J.; Bull, A.L.; Spelman, T.; Brett, J.; Richards, M.J. Diminishing surgical site infections in australia: time trends in infection rates, pathogens and antimicrobial resistance using a comprehensive victorian surveillance program, 2002–2013. Infect. Control Hosp. Epidemiol. 2015, 36, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Sahai, E.A.; Yeo, M.; Bosch, M.; Dewar, A.; Olson, M.F. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 2001, 3, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.F.; Kanaseki, T.; Sabirov, R.; Morishima, S.; Castro, J.; Bittner, C.X.; Maeno, E.; Ando-Akatsuka, Y.; Okada, Y. Apoptotic and necrotic blebs in epithelial cells display similar neck diameters but different kinase dependency. Cell Death Differ. 2003, 10, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Kim, K.Y.; Yoo, Y.J.; Oh, S.J.; Choi, J.H.; Kim, C.Y. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int. J. Antimicrob. Agents 2001, 18, 553–557. [Google Scholar] [CrossRef]
- Codling, C.E.; Hann, A.C.; Maillard, J.Y.; Russell, A.D. An investigation into the antimicrobial mechanisms of action of two contact lens biocides using electron microscopy. Contact Lens Anterior Eye 2005, 28, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Becerril, R.; Gómez-Lus, R.; Goni, P.; López, P.; Nerín, C. Combination of analytical and microbiological techniques to study the antimicrobial activity of a new active food packaging containing cinnamon or oregano against E. coli and S. aureus. Anal. Bioanal. Chem. 2007, 388, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Hecht, W. Methods for antimicrobial susceptibility testing of anaerobic bacteria: Approved standard. In Clinical and Laboratory Standards Institute (CLSI) Document M11-A8, 8th ed.; Wayne: Pennsylvania, PA, USA, 2012. [Google Scholar]
- Arzese, A.; Skerlavaj, B.; Tomasinsig, L.; Gennaro, R.; Zanetti, M. Antimicrobial activity of SMAP-29 against the Bacteroides fragilis group and clostridia. J. Antimicrob. Chemother. 2003, 52, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Kawakami, K.; Tohyama, K. Simultaneous evaluation of cell viability by neutral red, MTT and crystal violet staining assays of the same cells. Toxicol. In Vitro 1998, 12, 251–258. [Google Scholar] [CrossRef]
- Ngoc Ha, T.; Valentine, R.; Moratti, S.; Robinson, S.; Hanton, L.; Wormald, P.J. A blinded randomized controlled trial evaluating the efficacy of chitosan gel on ostial stenosis following endoscopic sinus surgery. Int. Forum Allergy Rhinol. 2013, 3, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Hydrogel systems for barriers and local drug delivery in the control of wound healing. J. Control. Release 1996, 39, 305–313. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, J.; Chen, L.; Yu, L.; Xu, J.; Ding, J. Biodegradable and thermoreversible PCLA–PEG–PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials 2011, 32, 4725–4736. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.D.; McConnell, M.A.; Fitzpatrick, C.; Mros, S.; Williams, G.; Wormald, P. J.; Moratti, S.C.; Hanton, L.R. Characterization of the in vivo host response to a bi-labeled chitosan-dextran based hydrogel for postsurgical adhesion prevention. J. Biomed. Mater. Res. Part A 2014. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, M.; Brooks, H.J.L.; Moratti, S.C.; Hanton, L.R.; Cabral, J.D. Reducing the Oxidation Level of Dextran Aldehyde in a Chitosan/Dextran-Based Surgical Hydrogel Increases Biocompatibility and Decreases Antimicrobial Efficacy. Int. J. Mol. Sci. 2015, 16, 13798-13814. https://doi.org/10.3390/ijms160613798
Chan M, Brooks HJL, Moratti SC, Hanton LR, Cabral JD. Reducing the Oxidation Level of Dextran Aldehyde in a Chitosan/Dextran-Based Surgical Hydrogel Increases Biocompatibility and Decreases Antimicrobial Efficacy. International Journal of Molecular Sciences. 2015; 16(6):13798-13814. https://doi.org/10.3390/ijms160613798
Chicago/Turabian StyleChan, Maggie, Heather J. L. Brooks, Stephen C. Moratti, Lyall R. Hanton, and Jaydee D. Cabral. 2015. "Reducing the Oxidation Level of Dextran Aldehyde in a Chitosan/Dextran-Based Surgical Hydrogel Increases Biocompatibility and Decreases Antimicrobial Efficacy" International Journal of Molecular Sciences 16, no. 6: 13798-13814. https://doi.org/10.3390/ijms160613798
APA StyleChan, M., Brooks, H. J. L., Moratti, S. C., Hanton, L. R., & Cabral, J. D. (2015). Reducing the Oxidation Level of Dextran Aldehyde in a Chitosan/Dextran-Based Surgical Hydrogel Increases Biocompatibility and Decreases Antimicrobial Efficacy. International Journal of Molecular Sciences, 16(6), 13798-13814. https://doi.org/10.3390/ijms160613798