Surface Modification of ZnO Nanorods with Hamilton Receptors
Abstract
:1. Introduction


2. Results and Discussion
2.1. Synthesis of Building Blocks


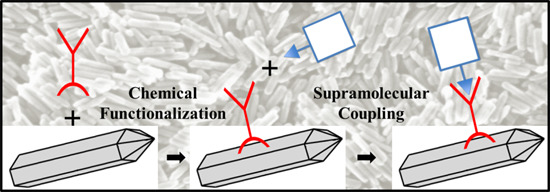
2.2. Chemical Surface Functionalization




2.3. Supramolecular Self-Assembly



3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Whitesides, G.M.; Grzybowski, B. Self-Assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Ikkala, O.; ten Brinke, G. Functional materials based on self-assembly of polymeric supramolecules. Science 2002, 295, 2407–2409. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. Toward self-organization and complex matter. Science 2002, 295, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Pachón, L.D.; Rothenberg, G. Transition-metal nanoparticles: Synthesis, stability and the leaching issue. Appl. Organomet. Chem. 2008, 22, 288–299. [Google Scholar] [CrossRef]
- Garnweitner, G. In-situ vs. post-synthetic stabilization of metal oxide nanoparticles. In The Delivery of Nanoparticles; Hashim, A.A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 71–92. [Google Scholar]
- Segets, D.; Marczak, R.; Schäfer, S.; Paula, C.; Gnichwitz, J.-F.; Hirsch, A.; Peukert, W. Experimental and theoretical studies of the colloidal stability of nanoparticles—A general interpretation based on stability maps. ACS Nano 2011, 5, 4658–4669. [Google Scholar] [CrossRef] [PubMed]
- Shenhar, R.; Rotello, V. Nanoparticles: Scaffolds and building blocks. Acc. Chem. Res. 2003, 36, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Gnichwitz, J.-F.; Marczak, R.; Werner, F.; Lang, N.; Jux, N.; Guldi, D.M.; Peukert, W.; Hirsch, A. Efficient synthetic access to cationic dendrons and their application for ZnO nanoparticles surface functionalization: New building blocks for dye-sensitized solar cells. J. Am. Chem. Soc. 2010, 132, 17910–17920. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Shenton, W.; Li, M.; Connolly, S.; Fitzmaurice, D. Biologically programmed nanoparticle assembly. Adv. Mater. 2000, 12, 147–150. [Google Scholar] [CrossRef]
- Boal, A.K.; Ilhan, F.; DeRouchey, J.E.; Thurn-Albrecht, T.; Russell, T.P.; Rotello, V.M. Self-assembly of nanoparticles into structured spherical and network aggregates. Nature 2000, 404, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.H.; Xing, H.; Lu, Y. DNA as a powerful tool for morphology control, spatial positioning and dynamic assembly of nanoparticles. Acc. Chem. Res. 2014, 47, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem. Int. Ed. Engl. 2001, 40, 4128–4158. [Google Scholar] [CrossRef]
- Chang, S.-K.; Hamilton, A.D. Molecular recognition of biologically interesting substrates: Synthesis of an artificial receptor for barbiturates employing six hydrogen bonds. J. Am. Chem. Soc. 1988, 110, 1318–1319. [Google Scholar] [CrossRef]
- Chang, S.-K.; Engen, D.V.; Fan, E.; Hamilton, A.D. Hydrogen bonding and molecular recognition: Synthetic, complexation, and structural studies on barbiturate binding to an artificial receptor. J. Am. Chem. Soc. 1991, 113, 7640–7645. [Google Scholar] [CrossRef]
- Dethlefs, C.; Eckelmann, J.; Kobarg, H.; Weyrich, T.; Brammer, S.; Näther, C.; Lüning, U. Determination of binding Constants of hydrogen-bonded complexes by ITC, NMR CIS, and NMR diffusion experiments. Eur. J. Org. Chem. 2011, 2011, 2066–2074. [Google Scholar] [CrossRef]
- Dirksen, A.; Hahn, U.; Schwanke, F.; Nieger, M.; Reek, J.N.H.; Vögtle, F.; de Cola, L. Multiple Recognition of barbiturate guests by hamilton-receptor-functionalized dendrimers. Chem. Eur. J. 2004, 10, 2036–2047. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.; Bauer, W.; Hirsch, A. Complete self-assembly of discrete supramolecular dendrimers. Angew. Chem. Int. Ed. Engl. 2005, 44, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Hager, K.; Hartnagel, U.; Hirsch, A. Supramolecular dendrimers self-assembled from dendritic fullerene ligands and a homotritopic hamilton receptor. Eur. J. Org. Chem. 2007, 2007, 1942–1956. [Google Scholar] [CrossRef]
- Binder, W.H.; Kluger, C.; Straif, C.J.; Friedbacher, G. Directed nanoparticle binding onto microphase-separated block copolymer thin films. Macromolecules 2005, 38, 9405–9410. [Google Scholar] [CrossRef]
- Grimm, F.; Ulm, N.; Gröhn, F.; Düring, J.; Hirsch, A. Self-assembly of supramolecular architectures and polymers by orthogonal metal complexation and hydrogen-bonding motifs. Chem. Eur. J. 2011, 17, 9478–9488. [Google Scholar] [CrossRef] [PubMed]
- Zirbs, R.; Kienberger, F.; Hinterdorfer, P.; Binder, W.H. Directed assembly of Au nanoparticles onto planar surfaces via multiple hydrogen bonds. Langmuir 2005, 21, 8414–8421. [Google Scholar] [CrossRef] [PubMed]
- Motesharei, K.; Myles, D.C. Molecular recognition on functionalized self-assembled monolayers of alkanethiols on gold. J. Am. Chem. Soc. 1998, 120, 7328–7336. [Google Scholar] [CrossRef]
- Ema, T.; Okuda, K.; Watanabe, S.; Yamasaki, T.; Minami, T.; Esipenko, N.A.; Anzenbacher, P., Jr. Selective anion sensing by chiral macrocyclic receptors with multiple hydrogen-bonding sites. Org. Lett. 2014, 16, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Tron, A.; Thornton, P.J.; Rocher, M.; Jacquot de Rouville, H.-P.; Desvergne, J.-P.; Kauffmann, B.; Buffeteau, T.; Cavagnat, D.; Tucker, J.H.; McClenaghan, N.D. Formation of a hydrogen-bonded barbiturate [2]-rotaxane. Org. Lett. 2014, 16, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Tecilla, P.; Dixon, R.P.; Slobodkin, G.; Alavi, D.S.; Waldeck, D.H.; Hamilton, A.D. Hydrogen-bonding self-assembly of multichromophore structures. J. Am. Chem. Soc. 1990, 112, 9408–9410. [Google Scholar]
- Dirksen, A.; Kleverlaan, C.J.; Reek, J.N.H.; de Cola, L. Ultrafast photoinduced electon transfer within a self-assembled donor-acceptor system. J. Phys. Chem. A 2005, 109, 5248–5256. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Schmidt, J.; Stolte, M.; Wortmann, R. Hydrogen-bond-directed head-to-tail orientation of dipolar merocyanine dyes: A strategy for the design of electrooptical materials. Angew. Chem. Int. Ed. Engl. 2006, 45, 3842–3846. [Google Scholar] [CrossRef] [PubMed]
- Wessendorf, F.; Gnichwitz, J.-F.; Sarova, G.H.; Hager, K.; Hartnagel, U.; Guldi, D.M.; Hirsch, A. Implementation of a hamilton-receptor-based hydrogen-bonding motif toward a new electron donor-acceptor prototype: Electron vs. energy transfer. J. Am. Chem. Soc. 2007, 129, 16057–16071. [Google Scholar] [CrossRef] [PubMed]
- Gnichwitz, J.-F.; Wielopolski, M.; Hartnagel, K.; Hartnagel, U.; Guldi, D.M.; Hirsch, A. Cooperativity and tunable excited state deactivation: Modular self-assembly of depsipeptide dendrons on a hamilton receptor modified porphyrin platform. J. Am. Chem. Soc. 2008, 130, 8491–8501. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.; Zeininger, L.; Hauke, F.; Hirsch, A. A supramolecular approach for the facile solubilization and separation of covalently functionalized single-walled carbon nanotubes. Chem. Eur. J. 2014, 20, 2537–2541. [Google Scholar] [CrossRef] [PubMed]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T. M.; Zuilhof, H. Covalent surface modification of oxide surfaces. Angew. Chem. Int. Ed. Engl. 2014, 53, 6322–6356. [Google Scholar] [CrossRef] [PubMed]
- Schönamsgruber, J.; Zeininger, L.; Hirsch, A. Grafting perylenes to ZnO nanoparticles. Chem. Eur. J. 2014, 20, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Voigt, M.; Klaumünzer, M.; Thiem, H.; Peukert, W. Detailed analysis of the growth kinetics of ZnO nanorods in methanol. J. Phys. Chem. C 2010, 114, 6243–6249. [Google Scholar] [CrossRef]
- Sun, Y.; Rogers, J.A. Inorganic semiconductors for flexible electronics. Adv. Mater. 2007, 19, 1897–1916. [Google Scholar] [CrossRef]
- Schaeffler, G.; Fuhr, O.; Fenske, D.; Lehn, J.-M. Self-assembly of a highly organized, hexameric supramolecular architecture: Formation, structure and properties. Chem. Eur. J. 2014, 20, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Portilla, L.; Halik, M. Smoothly tunable surface properties of aluminum oxide core-shell nanoparticles by a mixed-ligand approach. ACS Appl. Mater. Interfaces 2014, 6, 5977–5982. [Google Scholar] [CrossRef]
- Iacazio, G.; Périssol, C.; Faure, B. A new tannase substrate for spectrophotometric assay. J. Microbiol. Methods 2000, 42, 209–214. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeininger, L.; Klaumünzer, M.; Peukert, W.; Hirsch, A. Surface Modification of ZnO Nanorods with Hamilton Receptors. Int. J. Mol. Sci. 2015, 16, 8186-8200. https://doi.org/10.3390/ijms16048186
Zeininger L, Klaumünzer M, Peukert W, Hirsch A. Surface Modification of ZnO Nanorods with Hamilton Receptors. International Journal of Molecular Sciences. 2015; 16(4):8186-8200. https://doi.org/10.3390/ijms16048186
Chicago/Turabian StyleZeininger, Lukas, Martin Klaumünzer, Wolfgang Peukert, and Andreas Hirsch. 2015. "Surface Modification of ZnO Nanorods with Hamilton Receptors" International Journal of Molecular Sciences 16, no. 4: 8186-8200. https://doi.org/10.3390/ijms16048186
APA StyleZeininger, L., Klaumünzer, M., Peukert, W., & Hirsch, A. (2015). Surface Modification of ZnO Nanorods with Hamilton Receptors. International Journal of Molecular Sciences, 16(4), 8186-8200. https://doi.org/10.3390/ijms16048186