Single-Stage Operation of Hybrid Dark-Photo Fermentation to Enhance Biohydrogen Production through Regulation of System Redox Condition: Evaluation with Real-Field Wastewater
Abstract
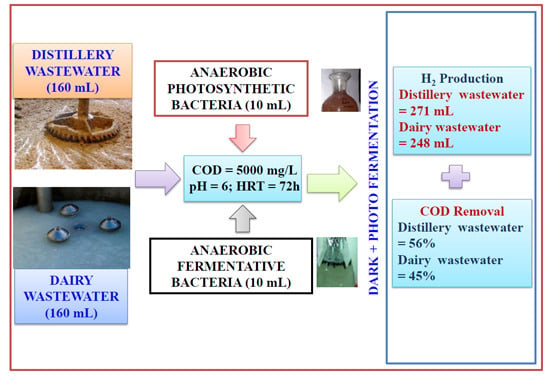
:1. Introduction
2. Result and Discussion
2.1. Bio-Hydrogenesis
2.1.1. Dark-Fermentation (DFS)

2.1.2. Hybrid Dark-Photo Fermentation
2.2. Pigments and Biomass



2.3. Total Volatile Fatty Acids and Composition


2.4. Substrate Degradation

2.5. Bio-Electrocatalytic Analysis
| Time (h) | OC (µA) | RC (µA) | βc (V/dec) | βa (V/dec) | Rp (kΩ) | |
|---|---|---|---|---|---|---|
| EDi | 0 | 0.25 | 0.23 | 0.219 | 0.408 | 15,216 |
| 24 | 0.24 | 0.29 | 0.263 | 0.625 | 11,473 | |
| 48 | 0.27 | 0.40 | 0.157 | 0.352 | 14,734 | |
| 72 | 0.24 | 0.37 | 0.165 | 0.377 | 15,173 | |
| CDi | 0 | 0.12 | 0.07 | 0.257 | 0.544 | 15,038 |
| 24 | 0.10 | 0.10 | 0.121 | 0.557 | 16,325 | |
| 48 | 0.08 | 0.08 | 0.059 | 0.667 | 15,440 | |
| 72 | 0.06 | 0.06 | 0.070 | 0.632 | 15,290 | |
| EDa | 0 | 0.07 | 0.09 | 0.683 | 0.158 | 16,430 |
| 24 | 0.06 | 0.06 | 0.205 | 0.622 | 17,580 | |
| 48 | 0.041 | 0.055 | 0.165 | 0.607 | 12,300 | |
| 72 | 0.042 | 0.053 | 0.155 | 0.726 | 16,480 | |
| CDa | 0 | 0.067 | 0.081 | 1.770 | 0.576 | 13,330 |
| 24 | 0.060 | 0.064 | 0.212 | 0.584 | 18,890 | |
| 48 | 0.054 | 0.060 | 0.192 | 0.608 | 18,136 | |
| 72 | 0.050 | 0.064 | 0.184 | 0.681 | 17,020 |

3. Experimental Section
3.1. Biocatalyst
3.1.1. Anaerobic Culture
3.1.2. Photosynthetic Culture
3.2. Wastewater
3.2.1. Dairy Wastewater
3.2.2. Distillery Wastewater
3.3. Experimental Methodology

3.4. Analytical Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hallenbeck, P.C.; Abo-Hashesh, M.; Ghosh, D. Strategies for improving biological hydrogen production. Bioresour. Technol. 2012, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Chang, J.-S.; Hallenbeck, P.C.; Larroche, C. Biohydrogen; Elsevier: San Diego, CA, USA, 2013. [Google Scholar]
- Venkata Mohan, S.; Lalit Babu, V.; Sarma, P.N. Anaerobic biohydrogen production from dairy wastewater treatment in sequencing batch reactor (AnSBR): Effect of organic loading rate. Enzym. Microb. Technol. 2007, 41, 506–515. [Google Scholar] [CrossRef]
- Venkata Mohan, S. Harnessing of biohydrogen from wastewater treatment using mixed fermentative consortia: Process evaluation towards optimization. Int. J. Hydrog. Energy 2009, 34, 7460–7474. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Mohan, S.V.; Sarma, P. Utilizing acid-rich effluents of fermentative hydrogen production process as substrate for harnessing bioelectricity: An integrative approach. Int. J. Hydrog. Energy 2010, 35, 3440–3449. [Google Scholar] [CrossRef]
- Babu, M.L.; Subhash, G.V.; Sarma, P.; Mohan, S.V. Bio-electrolytic conversion of acidogenic effluents to biohydrogen: An integration strategy for higher substrate conversion and product recovery. Bioresour. Technol. 2013, 133, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Nikhil, G.; Mohan, S.V.; Swamy, Y. Applied potentials regulate recovery of residual hydrogen from acid-rich effluents: Influence of biocathodic buffer capacity over process performance. Bioresour. Technol. 2015. [Google Scholar] [CrossRef]
- Escapa, A.; San Martin, M.I.; Moran, A. Potential use of microbial electrolysis cells (MECS) in domestic wastewater treatment plants for energy recovery. Front. Energy Res. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Thavasi, V.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Klimov, V.V.; Ramakrishna, S.; Los, D.A.; Mimuro, M.; Nishihara, H.; Carpentier, R. Photosynthetic hydrogen production. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 101–113. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Ghosh, D. Advances in fermentative biohydrogen production: The way forward? Trends Biotechnol. 2009, 27, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Venkata Mohan, S. Enhanced bio-hydrogenesis by co-culturing photosynthetic bacteria with acidogenic process: Augmented dark-photo fermentative hybrid system to regulate volatile fatty acid inhibition. Int. J. Hydrog. Energy 2014, 39, 7604–7615. [Google Scholar] [CrossRef]
- Sasikala, C.; Ramana, C.V. Biotechnological potentials of anoxygenic phototrophic bacteria. I. Production of single cell protein, vitamins, ubiquinones, hormones, and enzymes and use in waste treatment. Adv. Appl. Microbiol. 1995, 41, 173–226. [Google Scholar] [PubMed]
- Singh, S.; Srivastava, S.; Singh, V.; Block, D.; Veziroglu, T. Photoproduction of hydrogen by rhodopseudomonas at the expense of cellulose: Development of two-stage bioreactor, hydrogen energy progress X: Volume 2. In Proceedings of the 10th World Hydrogen Energy Conference,Cocoa Beach, FL,USA, 20–24 June 1994; pp. 959–963.
- Tao, Y.; Chen, Y.; Wu, Y.; He, Y.; Zhou, Z. High hydrogen yield from a two-step process of dark-and photo-fermentation of sucrose. Int. J. Hydrog. Energy 2007, 32, 200–206. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Mulcahy, D. Impact of carbon and nitrogen sources on hydrogen production by a newly isolated Clostridium butyricum w5. Int. J. Hydrog. Energy 2008, 33, 4998–5005. [Google Scholar] [CrossRef]
- Mohan, S.V.; Mohanakrishna, G.; Goud, R.K.; Sarma, P. Acidogenic fermentation of vegetable based market waste to harness biohydrogen with simultaneous stabilization. Bioresour. Technol. 2009, 100, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Sharma, A.; Singh, B. Study of physio-chemical characteristics and biological treatment of molasses-based distillery effluent. Int. J. Bioassays 2013, 2, 612–615. [Google Scholar]
- Nandy, T.; Shastry, S.; Kaul, S. Wastewater management in a cane molasses distillery involving bioresource recovery. J. Environ. Manag. 2002, 65, 25–38. [Google Scholar] [CrossRef]
- Hawkes, F.; Dinsdale, R.; Hawkes, D.; Hussy, I. Sustainable fermentative hydrogen production: Challenges for process optimisation. Int. J. Hydrog. Energy 2002, 27, 1339–1347. [Google Scholar] [CrossRef]
- Mohan, S.V.; Agarwal, L.; Mohanakrishna, G.; Srikanth, S.; Kapley, A.; Purohit, H.J.; Sarma, P. Firmicutes with iron dependent hydrogenase drive hydrogen production in anaerobic bioreactor using distillery wastewater. Int. J. Hydrog. Energy 2011, 36, 8234–8242. [Google Scholar] [CrossRef]
- Lee, C.-M.; Chen, P.-C.; Wang, C.-C.; Tung, Y.-C. Photohydrogen production using purple nonsulfur bacteria with hydrogen fermentation reactor effluent. Int. J. Hydrog. Energy 2002, 27, 1309–1313. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Mohan, S.V. Multiple process integrations for broad perspective analysis of fermentative H2 production from wastewater treatment: Technical and environmental considerations. Appl. Energy 2013, 107, 244–254. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Madigan, M.T.; Bauer, C.E. Anoxygenic Photosynthetic Bacteria; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Clayton, R.K. Spectroscopic analysis of bacteriochlorophylls in vitro and in vivo. Photochem. Photobiol. 1966, 5, 669–677. [Google Scholar] [CrossRef]
- Fujimori, E. Bacteriochlorophyll pheophytinization in chromatophores and subchromatophores from Rhodospirillum rubrum. Biochim. Biophys. Acta Bioenerg. 1969, 180, 360–367. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hamano, T.; Akiyama, M.; Watanabe, T.; Inoue, K.; Oh-oka, H.; Amesz, J.; Yamamura, M.; Kise, H. Light-independent isomerization of bacteriochlorophyll g to chlorophyll a catalyzed by weak acid in vitro. Anal. Chim. Acta 1998, 365, 199–203. [Google Scholar] [CrossRef]
- Ren, N.-Q.; Liu, B.-F.; Ding, J.; Guo, W.-Q.; Cao, G.-L.; Xie, G.-J. The effect of butyrate concentration on photo-hydrogen production from acetate by Rhodopseudomonas faecalis RLD-53. Int. J. Hydrog. Energy 2008, 33, 5981–5985. [Google Scholar] [CrossRef]
- Abo-Hashesh, M.; Desaunay, N.; Hallenbeck, P.C. High yield single stage conversion of glucose to hydrogen by photofermentation with continuous cultures of Rhodobacter capsulatus JP91. Bioresour. Technol. 2013, 128, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Venkata Mohan, S.; Prathima Devi, M.; Peri, D.; Sarma, P.N. Acetate and butyrate as substrates for hydrogen production through photo-fermentation: Process optimization and combined performance evaluation. Int. J. Hydrog. Energy 2009, 34, 7513–7522. [Google Scholar] [CrossRef]
- Liu, Y.; Ghosh, D.; Hallenbeck, P.C. Biological reformation of ethanol to hydrogen by Rhodopseudomonas palustris CGA009. Bioresour. Technol. 2015, 176, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Babu, V.L.; Sarma, P. Effect of various pretreatment methods on anaerobic mixed microflora to enhance biohydrogen production utilizing dairy wastewater as substrate. Bioresour. Technol. 2008, 99, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Venkata Mohan, S. Microalgal community and their growth conditions influence biohydrogen production during integration of dark-fermentation and photo-fermentation processes. Int. J. Hydrog. Energy 2011, 36, 12211–12219. [Google Scholar] [CrossRef]
- Eaton, A.D.; Franson, M.A.H.; Association, A.P.H.; Association, A.W.W.; Federation, W.E. Standard Methods for the Examination of Water & Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra, R.; Nikhil, G.N.; Mohan, S.V. Single-Stage Operation of Hybrid Dark-Photo Fermentation to Enhance Biohydrogen Production through Regulation of System Redox Condition: Evaluation with Real-Field Wastewater. Int. J. Mol. Sci. 2015, 16, 9540-9556. https://doi.org/10.3390/ijms16059540
Chandra R, Nikhil GN, Mohan SV. Single-Stage Operation of Hybrid Dark-Photo Fermentation to Enhance Biohydrogen Production through Regulation of System Redox Condition: Evaluation with Real-Field Wastewater. International Journal of Molecular Sciences. 2015; 16(5):9540-9556. https://doi.org/10.3390/ijms16059540
Chicago/Turabian StyleChandra, Rashmi, G. N. Nikhil, and S. Venkata Mohan. 2015. "Single-Stage Operation of Hybrid Dark-Photo Fermentation to Enhance Biohydrogen Production through Regulation of System Redox Condition: Evaluation with Real-Field Wastewater" International Journal of Molecular Sciences 16, no. 5: 9540-9556. https://doi.org/10.3390/ijms16059540
APA StyleChandra, R., Nikhil, G. N., & Mohan, S. V. (2015). Single-Stage Operation of Hybrid Dark-Photo Fermentation to Enhance Biohydrogen Production through Regulation of System Redox Condition: Evaluation with Real-Field Wastewater. International Journal of Molecular Sciences, 16(5), 9540-9556. https://doi.org/10.3390/ijms16059540