Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes
Abstract
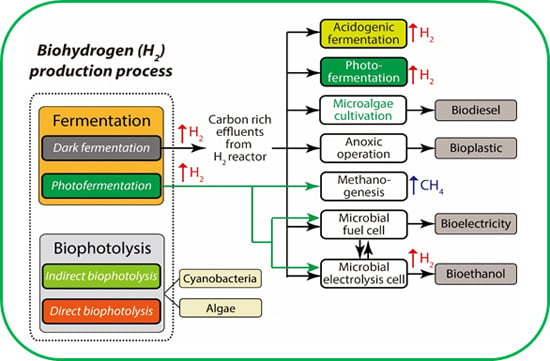
:1. Introduction

2. Biohydrogen
2.1. Diversity of Microorganisms as H2 Producing Biocatalysts


2.2. Water-Splitting Photosynthesis (Biophotolysis)

2.3. Anoxygenic Photofermentation
2.4. Dark Fermentation
2.5. Microbial Electrolysis Cells (Electrofermentation)
3. Effects of Operational Factors for H2 Production
3.1. Temperature
3.2. pH
3.3. Nutrients
3.4. Hydraulic Retention Time
3.5. Partial Pressure of H2
4. Economic Feasibility and Technical Challenges
| Type of Bioprocess | Technical Challenges |
|---|---|
| Dark fermentation |
|
| Photofermentation |
|
| Direct biophotolysis |
|
| Indirect biophotolysis |
|
5. Strategies to Enhance the Efficiency of the Process
5.1. Integration of Approaches
| Substrate | First Stage | Second Stage | Reference | ||
|---|---|---|---|---|---|
| Process Type | Yield | Process Type | Yield | ||
| Cornstalks | Hydrogen (DF) | 58.0 mL/g | Methane (DF) | 200.9 mL/g | [93] |
| Rice straw | Hydrogen (DF) | 20 mL/g | Methane (DF) | 260 mL/g | [94] |
| Water hyacinth | Hydrogen (DF) | 38.2 mmol H2/L/day | Methane (DF) | 29 mmol CH4/L/d | [95] |
| Water hyacinth | Hydrogen (DF) | 51.7 mL of H2/g of TVS | Methane (DF) | 43.4 mL of CH4/g of TVS | [96] |
| Laminaria japonica | Hydrogen (DF) | 115.2 mL of H2/g | Methane (DF) | 329.8 mL of CH4/g | [97] |
| Cassava wastewater | Hydrogen (DF) | 54.22 mL of H2/g | Methane (DF) | 164.87 mL of CH4/g | [98] |
| Microalgal biomass | Hydrogen (DF) | 135 ± 3.11 mL of H2/g/VS | Methane (DF) | 414 ± 2.45 mL of CH4/g/VS | [99] |
| Glucose | Hydrogen (DF) | 1.20 mmol | Hydrogen (PF) | 5.22 mmol | [100] |
| Cheese whey wastewater | Hydrogen (DF) | 2.04 mol | Hydrogen (PF) | 2.69 mol | [101] |
| Vegetable waste | Hydrogen (DF) | 12.61 mmol H2/day | Electricity (DF) | 111.76 mW/m2 | [87] |
| Fruit juice industry wastewater | Hydrogen (DF) | 1.4 mol H2/mol hexose | Electricity (DF) | 0.55 W/m2 | [102] |
| Corn stover lignocellulose | Hydrogen (DF) | 1.67 mol H2/mol glucose | Hydrogen (MEC) | 1.00 L/L-d | [103] |
| Cellobiose | Hydrogen (DF) | 1.64 mol H2/mol glucose | Hydrogen (MEC) | 0.96 L/L-d | [104] |
| Distillery spent wash | Hydrogen (DF) | 39.8 L | Bioplastic | 40% dry cell weight | [105] |
| Food waste | Hydrogen (DF) | 3.18 L | Bioplastic | 36% dry cell weight | [106] |
| Pea shells | Hydrogen (DF) | 5.2 L of H2 from 4 L | Bioplastic | 1685 mg of PHB/L | [107] |
| Food waste | Hydrogen (DF) | 69.94 mmol | Lipid | 26.4% dry cell weight | [108] |
| Olive oil mill wastewater | Hydrogen (DF) | 196.2 mL/g | Biopolymer | 8.9% dry cell weight | [109] |
| Molasses wastewater | Hydrogen (DF) | 130.57 mmol | Ethanol | 379.3 mg/L | [110] |
| Food waste | Bioelectricity | 85.2 mW/m2 | Hydrogen (DF) | 0.91 L | [39] |
| Starch hydrolysate | Hydrogen (DF) | 5.40 mmol H2/g of COD | Hydrogen (PF) | 10.72 mmol H2/g of COD | [111] |
| Sucrose | Hydrogen (DF) | 0.98 ± 0.32 mol H2/mol | Hydrogen (PF) | 4.48 ± 0.23 mol H2/mol | [112] |
| Glucose:xylose (9:1); Microalgae biomass | Hydrogen (DF) | 250 mL/L/h; 2.78 mol H2/mol | Mixotropic microalgae cultivation | 205 mL/L/h; 1.12 g of biomass/g of COD | [113] |
5.2. Photobiological Process
5.3. Biodegradable Plastics
5.4. Electrically Driven Biohydrogenesis from Acid-Rich Effluents
5.5. Bioaugmentation

5.6. Utilization of Organic Wastes as a Fermentable Substrate
5.7. Pretreatment of Substrates and Biocatalysts
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goldemberg, J. nmental and ecological dimensions of biofuels. In. In Proceedings of the Conference on the Ecological Dimensions of Biofuels, Washington, DC, USA, 10 March 2008.
- Venkata Mohan, S.; Pandey, A. Biohydrogen production: An introduction. In Biohydrogen; Pandey, A., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–24. [Google Scholar]
- Lee, S.J.; Lee, S.J.; Lee, D.W. Design and development of synthetic microbial platform cells for bioenergy. Front. Microbiol. 2013, 4, 92. [Google Scholar] [PubMed]
- Nouni, M.R. Hydrogen energy and fuel cell technology: Recent developments and future prospects in india. Renew. Energy Akshay Urja 2012, 5, 10–14. [Google Scholar]
- Bockris, J.O.M. The origin of ideas on a hydrogen economy and its solution to the decay of the environment. Int. J. Hydrog. Energy 2002, 27, 731–740. [Google Scholar] [CrossRef]
- Christopher, K.; Dimitrios, R. A review on exergy comparison of hydrogen production methods from renewable energy sources. Energy Environ. Sci. 2012, 5, 6640–6651. [Google Scholar] [CrossRef]
- Lipman, T. An overview of hydrogen production and storage systems with renewable hydrogen case studies. In Energy Efficiency and Renewable Energy Fuel Cell Technologies Program (US DOE Grant DE-FC3608GO18111A000); Clean Energy States Alliance: Oakland, CA, USA, 2011. [Google Scholar]
- Jong, W.D. Sustainable hydrogen production by thermochemical biomass processing. Chapter 2008, 6, 185–225. [Google Scholar]
- Chen, W.H.; Syu, Y.J. Hydrogen production from water gas shift reaction in a high gravity (Higee) environment using a rotating packed bed. Int. J. Hydrog. Energy 2010, 35, 10179–10189. [Google Scholar] [CrossRef]
- Hydrogen generation market-by merchant & captive type, distributed & centralized generation. Report Code: EP 1708. Available online: http://www.marketsandmarkets.com/Market-Reports/hydrogen-generation-market-494.html (accessed on 12 April 2015).
- Hallenbeck, P.C.; Benemann, J.R. Biological hydrogen production; fundamentals and limiting processes. Int. J. Hydrog. Energy 2002, 27, 1185–1193. [Google Scholar] [CrossRef]
- Hallenbeck, P. Hydrogen production by cyanobacteria. In Microbial Technologies in Advanced Biofuels Production; Hallenbeck, P.C., Ed.; Springer: New York, NY, USA, 2012; pp. 15–28. [Google Scholar]
- Boichenko, V.A.; Greenbaum, E.; Seibert, M. Hydrogen production by photosynthetic microorganisms. In Molecular to Global Photosynthesis; Imperial College Press: London, UK, 2004; pp. 397–451. [Google Scholar]
- Das, D. Advances in biohydrogen production processes: An approach towards commercialization. Int. J. Hydrog. Energy 2009, 34, 7349–7357. [Google Scholar] [CrossRef]
- Mullai, P.; Rene, E.R.; Sridevi, K. Biohydrogen production and kinetic modeling using sediment microorganisms of pichavaram mangroves, India. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Domiguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Wan, W. Factors influencing fermentative hydrogen production: A review. Int. J. Hydrog. Energy 2009, 34, 799–811. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Chandrasekhar, K.; Chiranjeevi, P.; Babu, P.S. Biohydrogen production from wastewater. In Biohydrogen; Pandey, A., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 223–257. [Google Scholar]
- Melis, A.; Zhang, L.; Forestier, M.; Ghirardi, M.L.; Seibert, M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green Alga Chlamydomonas reinhardtii. Plant Physiol. 2000, 122, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.S.; Mayfield, S.P. Algae biofuels: Versatility for the future of bioenergy. Curr. Opin. Biotechnol. 2012, 23, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Melis, A. Photobiological hydrogen production: Recent advances and state of the art. Bioresour. Technol. 2011, 102, 8403–8413. [Google Scholar] [CrossRef] [PubMed]
- Tekucheva, D.N.; Tsygankov, A.A. Coupled biological hydrogen-producing systems: A review. Prikl. Biokhimiia Mikrobiol. 2012, 48, 357–375. [Google Scholar]
- Huesemann, M.H.; Hausmann, T.S.; Carter, B.M.; Gerschler, J.J.; Benemann, J.R. Hydrogen generation through indirect biophotolysis in batch cultures of the nonheterocystous nitrogen-fixing cyanobacterium plectonema boryanum. Appl. Biochem. Biotechnol. 2010, 162, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Stöckel, J.; Min, H.; Sherman, L.A.; Pakrasi, H.B. High rates of photobiological H2 production by a cyanobacterium under aerobic conditions. Nat. Commun. 2010, 1, 139. [Google Scholar] [CrossRef] [PubMed]
- Melnicki, M.R.; Pinchuk, G.E.; Hill, E.A.; Kucek, L.A.; Fredrickson, J.K.; Konopka, A.; Beliaev, A.S. Sustained H2 production driven by photosynthetic water splitting in a unicellular cyanobacterium. MBio 2012, 3, e00197–e00112. [Google Scholar] [CrossRef] [PubMed]
- Kosourov, S.N.; Ghirardi, M.L.; Seibert, M. A truncated antenna mutant of chlamydomonas reinhardtii can produce more hydrogen than the parental strain. Int. J. Hydrog. Energy 2011, 36, 2044–2048. [Google Scholar] [CrossRef]
- Scoma, A.; Krawietz, D.; Faraloni, C.; Giannelli, L.; Happe, T.; Torzillo, G. Sustained H2 production in a chlamydomonas reinhardtii D1 protein mutant. J. Biotechnol. 2012, 157, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Esquível, M.G.; Amaro, H.M.; Pinto, T.S.; Fevereiro, P.S.; Malcata, F.X. Efficient H2 production via chlamydomonas reinhardtii. Trends Biotechnol. 2011, 29, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Ducat, D.C.; Sachdeva, G.; Silver, P.A. Rewiring hydrogenase-dependent redox circuits in cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 3941–3946. [Google Scholar] [CrossRef] [PubMed]
- Basak, N.; Das, D. The prospect of purple non-sulfur (PNS) photosynthetic bacteria for hydrogen production: The present state of the art. World J. Microbiol. Biotechnol. 2007, 23, 31–42. [Google Scholar] [CrossRef]
- Azwar, M.Y.; Hussain, M.A.; Abdul-Wahab, A.K. Development of biohydrogen production by photobiological, fermentation and electrochemical processes: A review. Renew. Sustain. Energy Rev. 2014, 31, 158–173. [Google Scholar] [CrossRef]
- Brentner, L.B.; Jordan, P.A.; Zimmerman, J.B. Challenges in developing biohydrogen as a sustainable energy source: Implications for a research agenda. Environ. Sci. Technol. 2010, 44, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Koku, H.; Eroğlu, İ.; Gündüz, U.; Yücel, M.; Türker, L. Aspects of the metabolism of hydrogen production by rhodobacter sphaeroides. Int. J. Hydrog. Energy 2002, 27, 1315–1329. [Google Scholar] [CrossRef]
- Ghosh, D.; Sobro, I.F.; Hallenbeck, P.C. Optimization of the hydrogen yield from single-stage photofermentation of glucose by rhodobacter capsulatus jp91 using response surface methodology. Bioresour. Technol. 2012, 123, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Tourigny, A.; Hallenbeck, P.C. Near stoichiometric reforming of biodiesel derived crude glycerol to hydrogen by photofermentation. Int. J. Hydrog. Energy 2012, 37, 2273–2277. [Google Scholar] [CrossRef]
- Ghosh, D.; Sobro, I.F.; Hallenbeck, P.C. Stoichiometric conversion of biodiesel derived crude glycerol to hydrogen: Response surface methodology study of the effects of light intensity and crude glycerol and glutamate concentration. Bioresour. Technol. 2012, 106, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.C.; Ghosh, D. Advances in fermentative biohydrogen production: The way forward? Trends Biotechnol. 2009, 27, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.; Venkata Mohan, S. Bio-electrohydrolysis as a pretreatment strategy to catabolize complex food waste in closed circuitry: Function of electron flux to enhance acidogenic biohydrogen production. Int. J. Hydrog. Energy 2014, 39, 11411–11422. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Venkata Mohan, S. Induced catabolic bio-electrohydrolysis of complex food waste by regulating external resistance for enhancing acidogenic biohydrogen production. Bioresour. Technol. 2014, 165, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Vardar-Schara, G.; Maeda, T.; Wood, T.K. Metabolically engineered bacteria for producing hydrogen via fermentation. Microb. Biotechnol. 2008, 1, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, J.; Bagley, D. Improving the yield from fermentative hydrogen production. Biotechnol. Lett. 2007, 29, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Chandrasekhar, K. Solid phase microbial fuel cell (SMFC) for harnessing bioelectricity from composite food waste fermentation: Influence of electrode assembly and buffering capacity. Bioresour. Technol. 2011, 102, 7077–7085. [Google Scholar]
- Nath, K.; Das, D. Modeling and optimization of fermentative hydrogen production. Bioresour. Technol. 2011, 102, 8569–8581. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. Sustainable and efficient biohydrogen production via electrohydrogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18871–18873. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.M.; Molenkamp, R.J.; Buisman, C.J.N. Performance of single chamber biocatalyzed electrolysis with different types of ion exchange membranes. Water Res. 2007, 41, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.C. Microbial paths to renewable hydrogen production. Biofuels 2011, 2, 285–302. [Google Scholar] [CrossRef]
- Clauwaert, P.; Verstraete, W. Methanogenesis in membraneless microbial electrolysis cells. Appl. Microbiol. Biotechnol. 2009, 82, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Ohmori, H.; Waki, M.; Ogino, A.; Tanaka, Y. Continuous hydrogen production from glucose by using extreme thermophilic anaerobic microflora. J. Biosci. Bioeng. 2009, 107, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrog. Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Tang, G.-L.; Huang, J.; Sun, Z.-J.; Tang, Q.-Q.; Yan, C.-H.; Liu, G.-Q. Biohydrogen production from cattle wastewater by enriched anaerobic mixed consortia: Influence of fermentation temperature and ph. J. Biosci. Bioeng. 2008, 106, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Fang, H.H.P. Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit. Rev. Environ. Sci. Technol. 2007, 37, 1–39. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chang, C.-C.; Hung, C.-H. Fermentative hydrogen production from starch using natural mixed cultures. Int. J. Hydrog. Energy 2008, 33, 2445–2453. [Google Scholar] [CrossRef]
- Maru, B.T.; Bielen, A.A.M.; Constantí, M.; Medina, F.; Kengen, S.W.M. Glycerol fermentation to hydrogen by thermotoga maritima: Proposed pathway and bioenergetic considerations. Int. J. Hydrog. Energy 2013, 38, 5563–5572. [Google Scholar] [CrossRef]
- De Vrije, T.C.P. Dark hydrogen fermentations. In Biomethane & Biohydrogen; Reith, J.H., Wijffels, R.H., Barten, H., Eds.; Smiet Offset: Hague, The Netherlands, 2003; pp. 103–123. [Google Scholar]
- Kiran Kumar, A.; Venkateswar Reddy, M.; Chandrasekhar, K.; Srikanth, S.; Venkata Mohan, S. Endocrine disruptive estrogens role in electron transfer: Bio-electrochemical remediation with microbial mediated electrogenesis. Bioresour. Technol. 2012, 104, 547–556. [Google Scholar]
- Venkata Mohan, S.; Chandrasekhar, K. Self-induced bio-potential and graphite electron accepting conditions enhances petroleum sludge degradation in bio-electrochemical system with simultaneous power generation. Bioresour. Technol. 2011, 102, 9532–9541. [Google Scholar]
- Antonopoulou, G.; Stamatelatou, K.; Venetsaneas, N.; Kornaros, M.; Lyberatos, G. Biohydrogen and methane production from cheese whey in a two-stage anaerobic process. Indust. Eng. Chem. Res. 2008, 47, 5227–5233. [Google Scholar] [CrossRef]
- Fan, Y.; Li, C.; Lay, J.-J.; Hou, H.; Zhang, G. Optimization of initial substrate and pH levels for germination of sporing hydrogen-producing anaerobes in cow dung compost. Bioresour. Technol. 2004, 91, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, S.; Sung, S.; Lay, J.J. Biohydrogen production as a function of ph and substrate concentration. Environ. Sci. Technol. 2001, 35, 4726–4730. [Google Scholar]
- Zhu, H.; Béland, M. Evaluation of alternative methods of preparing hydrogen producing seeds from digested wastewater sludge. Int. J. Hydrog. Energy 2006, 31, 1980–1988. [Google Scholar] [CrossRef]
- Özgür, E.; Afsar, N.; de Vrije, T.; Yücel, M.; Gündüz, U.; Claassen, P.A.M.; Eroglu, I. Potential use of thermophilic dark fermentation effluents in photofermentative hydrogen production by rhodobacter capsulatus. J. Clean. Prod. 2010, 18, S23–S28. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lay, C.H. Effects of carbonate and phosphate concentrations on hydrogen production using anaerobic sewage sludge microflora. Int. J. Hydrog. Energy 2004, 29, 275–281. [Google Scholar] [CrossRef]
- Wang, B.; Wan, W.; Wang, J. Effect of ammonia concentration on fermentative hydrogen production by mixed cultures. Bioresour. Technol. 2009, 100, 1211–1213. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-D.; Lee, K.-S.; Lo, Y.-C.; Chen, W.-M.; Wu, J.-F.; Lin, C.-Y.; Chang, J.-S. Batch and continuous biohydrogen production from starch hydrolysate by clostridium species. Int. J. Hydrog. Energy 2008, 33, 1803–1812. [Google Scholar] [CrossRef]
- Redwood, M.D.; Macaskie, L.E. A two-stage, two-organism process for biohydrogen from glucose. Int. J. Hydrog. Energy 2006, 31, 1514–1521. [Google Scholar] [CrossRef]
- Salerno, M.B.; Park, W.; Zuo, Y.; Logan, B.E. Inhibition of biohydrogen production by ammonia. Water Res. 2006, 40, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lay, C.H. Carbon/nitrogen-ratio effect on fermentative hydrogen production by mixed microflora. Int. J. Hydrog. Energy 2004, 29, 41–45. [Google Scholar] [CrossRef]
- Yadvika; Santosh; Sreekrishnan, T.R.; Kohli, S.; Rana, V. Enhancement of biogas production from solid substrates using different techniques-a review. Bioresour. Technol. 2004, 95, 1–10. [Google Scholar]
- Lin, C.Y.; Shei, S.H. Heavy metal effects on fermentative hydrogen production using natural mixed microflora. Int. J. Hydrog. Energy 2008, 33, 587–593. [Google Scholar] [CrossRef]
- Tsaousis, A.D.; Nývltová, E.; Šuták, R.; Hrdý, I.; Tachezy, J. A nonmitochondrial hydrogen production in naegleria gruberi. Genome Biol. Evol. 2014, 6, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Miyahara, T.; Noike, T. Effect of iron concentration on hydrogen fermentation. Bioresour. Technol. 2001, 80, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Venkata Mohan, S. Regulatory function of divalent cations in controlling the acidogenic biohydrogen production process. RSC Adv. 2012, 2, 6576–6589. [Google Scholar] [CrossRef]
- Karadag, D.; Puhakka, J.A. Enhancement of anaerobic hydrogen production by iron and nickel. Int. J. Hydrog. Energy 2010, 35, 8554–8560. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Shen, J. Hydrogen production in batch culture of mixed bacteria with sucrose under different iron concentrations. Int. J. Hydrog. Energy 2005, 30, 855–860. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J. Effect of temperature and iron concentration on the growth and hydrogen production of mixed bacteria. Int. J. Hydrog. Energy 2006, 31, 441–446. [Google Scholar] [CrossRef]
- Chang, F.Y.; Lin, C.Y. Calcium effect on fermentative hydrogen production in an anaerobic up-flow sludge blanket system. Water Sci. Technol. 2006, 54, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, F.R.; Hussy, I.; Kyazze, G.; Dinsdale, R.; Hawkes, D.L. Continuous dark fermentative hydrogen production by mesophilic microflora: Principles and progress. Int. J. Hydrog. Energy 2007, 32, 172–184. [Google Scholar] [CrossRef]
- Venkata Mohan, S. Waste to renewable energy: A sustainable and green approach towards production of biohydrogen by acidogenic fermentation. In Sustainable Biotechnology; Singh, O.V., Harvey, S.P., Eds.; Springer: Amsterdam, The Netherlands, 2010; pp. 129–164. [Google Scholar]
- Vijaya Bhaskar, Y.; Venkata Mohan, S.; Sarma, P.N. Effect of substrate loading rate of chemical wastewater on fermentative biohydrogen production in biofilm configured sequencing batch reactor. Bioresour. Technol. 2008, 99, 6941–6948. [Google Scholar]
- Ren, N.; Chen, Z.; Wang, A.; Hu, D. Removal of organic pollutants and analysis of mlss–cod removal relationship at different hrts in a submerged membrane bioreactor. Int. Biodeterior. Biodegrad. 2005, 55, 279–284. [Google Scholar] [CrossRef]
- Chen, W.-H.; Sung, S.; Chen, S.-Y. Biological hydrogen production in an anaerobic sequencing batch reactor: pH and cyclic duration effects. Int. J. Hydrog. Energy 2009, 34, 227–234. [Google Scholar] [CrossRef]
- Nath, K.; Das, D. Improvement of fermentative hydrogen production: Various approaches. Appl. Microbiol. Biotechnol. 2004, 65, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Van Groenestijn, J.W.; Hazewinkel, J.H.O.; Nienoord, M.; Bussmann, P.J.T. Energy aspects of biological hydrogen production in high rate bioreactors operated in the thermophilic temperature range. Int. J. Hydrog. Energy 2002, 27, 1141–1147. [Google Scholar]
- Abreu, A.A.; Karakashev, D.; Angelidaki, I.; Sousa, D.Z.; Alves, M.M. Biohydrogen production from arabinose and glucose using extreme thermophilic anaerobic mixed cultures. Biotechnol. Biofuels 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abo-Hashesh, M.; Hallenbeck, P.C. Microaerobic dark fermentative hydrogen production by the photosynthetic bacterium, rhodobacter capsulatus jp91. Int. J. Low Carbon Technol. 2012, 7, 97–103. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Venkata Mohan, S.; Sarma, P.N. Utilizing acid-rich effluents of fermentative hydrogen production process as substrate for harnessing bioelectricity: An integrative approach. Int. J. Hydrog. Energy 2010, 35, 3440–3449. [Google Scholar] [CrossRef]
- Gadhamshetty, V.; Sukumaran, A.; Nirmalakhandan, N. Review: Photoparameters in photofermentative biohydrogen production. J. Biosci. Bioeng. 2011, 41, 1–51. [Google Scholar]
- Keskin, T.; Abo-Hashesh, M.; Hallenbeck, P.C. Photofermentative hydrogen production from wastes. Bioresour. Technol. 2011, 102, 8557–8568. [Google Scholar] [CrossRef] [PubMed]
- Laurinavichene, T.V.; Belokopytov, B.F.; Laurinavichius, K.S.; Khusnutdinova, A.N.; Seibert, M.; Tsygankov, A.A. Towards the integration of dark- and photo-fermentative waste treatment. 4. Repeated batch sequential dark- and photofermentation using starch as substrate. Int. J. Hydrog. Energy 2012, 37, 8800–8810. [Google Scholar] [CrossRef]
- Laurinavichene, T.V.; Belokopytov, B.F.; Laurinavichius, K.S.; Tekucheva, D.N.; Seibert, M.; Tsygankov, A.A. Towards the integration of dark- and photo-fermentative waste treatment. 3. Potato as substrate for sequential dark fermentation and light-driven h2 production. Int. J. Hydrog. Energy 2010, 35, 8536–8543. [Google Scholar] [CrossRef]
- Belokopytov, B.F.; Laurinavichius, K.S.; Laurinavichene, T.V.; Ghirardi, M.L.; Seibert, M.; Tsygankov, A.A. Towards the integration of dark- and photo-fermentative waste treatment. 2. Optimization of starch-dependent fermentative hydrogen production. Int. J. Hydrog. Energy 2009, 34, 3324–3332. [Google Scholar] [CrossRef]
- Cheng, X.-Y.; Li, Q.; Liu, C.-Z. Coproduction of hydrogen and methane via anaerobic fermentation of cornstalk waste in continuous stirred tank reactor integrated with up-flow anaerobic sludge bed. Bioresour. Technol. 2012, 114, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-H.; Whang, L.-M.; Wu, C.-W.; Chung, M.-C. A two-stage bioprocess for hydrogen and methane production from rice straw bioethanol residues. Bioresour. Technol. 2012, 113, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-S.; Lay, C.-H.; Sen, B.; Chen, C.-C.; K, G.; Wu, J.-H.; Lin, C.-S.; Lin, C.-Y. Biohydrogen and biomethane from water hyacinth (eichhornia crassipes) fermentation: Effects of substrate concentration and incubation temperature. Int. J. Hydrog. Energy 2011, 36, 14195–14203. [Google Scholar] [CrossRef]
- Cheng, J.; Xie, B.; Zhou, J.; Song, W.; Cen, K. Cogeneration of H2 and CH4 from water hyacinth by two-step anaerobic fermentation. Int. J. Hydrog. Energy 2010, 35, 3029–3035. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, D.-H.; Shin, H.-S. Continuous fermentative hydrogen and methane production from laminaria japonica using a two-stage fermentation system with recycling of methane fermented effluent. Int. J. Hydrog. Energy 2012, 37, 15648–15657. [Google Scholar] [CrossRef]
- Intanoo, P.; Rangsanvigit, P.; Malakul, P.; Chavadej, S. Optimization of separate hydrogen and methane production from cassava wastewater using two-stage upflow anaerobic sludge blanket reactor (UASB) system under thermophilic operation. Bioresour. Technol. 2014, 173, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, N.; Kucuker, M.A.; Kuchta, K. Fermentative hydrogen and methane production from microalgal biomass (chlorella vulgaris) in a two-stage combined process. Appl. Energy 2014, 132, 108–117. [Google Scholar] [CrossRef]
- Chandra, R.; Venkata Mohan, S. Microalgal community and their growth conditions influence biohydrogen production during integration of dark-fermentation and photo-fermentation processes. Int. J. Hydrog. Energy 2011, 36, 12211–12219. [Google Scholar] [CrossRef]
- Rai, P.; Singh, S.P.; Asthana, R.K. Biohydrogen production from cheese whey wastewater in a two-step anaerobic process. Appl. Biochem. Biotechnol. 2012, 167, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez del Campo, A.; Cañizares, P.; Lobato, J.; Rodrigo, M.A.; Fernandez, F.J. Electricity production by integration of acidogenic fermentation of fruit juice wastewater and fuel cells. Int. J. Hydrog. Energy 2012, 37, 9028–9037. [Google Scholar] [CrossRef]
- Lalaurette, E.; Thammannagowda, S.; Mohagheghi, A.; Maness, P.-C.; Logan, B.E. Hydrogen production from cellulose in a two-stage process combining fermentation and electrohydrogenesis. Int. J. Hydrog. Energy 2009, 34, 6201–6210. [Google Scholar] [CrossRef]
- Lenin Babu, M.; Venkata Subhash, G.; Sarma, P.N.; Venkata Mohan, S. Bio-electrolytic conversion of acidogenic effluents to biohydrogen: An integration strategy for higher substrate conversion and product recovery. Bioresour. Technol. 2013, 133, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Amulya, K.; Venkateswar Reddy, M.; Venkata Mohan, S. Acidogenic spent wash valorization through polyhydroxyalkanoate (PHA) synthesis coupled with fermentative biohydrogen production. Bioresour. Technol. 2014, 158, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Venkateswar Reddy, M.; Amulya, K.; Rohit, M.V.; Sarma, P.N.; Venkata Mohan, S. Valorization of fatty acid waste for bioplastics production using bacillus tequilensis: Integration with dark-fermentative hydrogen production process. Int. J. Hydrog. Energy 2014, 39, 7616–7626. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Singh, M.; Kumar, P.; Purohit, H.J.; Kalia, V.C. Exploitation of defined bacterial cultures for production of hydrogen and polyhydroxybutyrate from pea-shells. Biomass Bioenergy 2012, 36, 218–225. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Prathima Devi, M. Fatty acid rich effluent from acidogenic biohydrogen reactor as substrate for lipid accumulation in heterotrophic microalgae with simultaneous treatment. Bioresour. Technol. 2012, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Ntaikou, I.; Kourmentza, C.; Koutrouli, E.C.; Stamatelatou, K.; Zampraka, A.; Kornaros, M.; Lyberatos, G. Exploitation of olive oil mill wastewater for combined biohydrogen and biopolymers production. Bioresour. Technol. 2009, 100, 3724–3730. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wang, B.; Zhou, Y.; Wang, D.-X.; Wang, Y.; Yue, L.-R.; Li, Y.-F.; Ren, N.-Q. Fermentative hydrogen production from molasses wastewater in a continuous mixed immobilized sludge reactor. Bioresour. Technol. 2012, 110, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-C.; Chen, S.-D.; Chen, C.-Y.; Huang, T.-I.; Lin, C.-Y.; Chang, J.-S. Combining enzymatic hydrolysis and dark-photo fermentation processes for hydrogen production from starch feedstock: A feasibility study. Int. J. Hydrog. Energy 2008, 33, 5224–5233. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Chen, C.-Y.; Lee, C.-M.; Chang, J.-S. Sequential dark-photo fermentation and autotrophic microalgal growth for high-yield and co2-free biohydrogen production. Int. J. Hydrog. Energy 2010, 35, 10944–10953. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chang, C.-Y.; Liao, Q.; Zhu, X.; Liao, C.-F.; Chang, J.-S. Biohydrogen production by a novel integration of dark fermentation and mixotrophic microalgae cultivation. Int. J. Hydrog. Energy 2013, 38, 15807–15814. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Venkata Mohan, S. Multiple process integrations for broad perspective analysis of fermentative h2 production from wastewater treatment: Technical and environmental considerations. Appl. Energy 2013, 107, 244–254. [Google Scholar] [CrossRef]
- Bala Amutha, K.; Murugesan, A.G. Biological hydrogen production by the algal biomass Chlorella vulgaris MSU 01 strain isolated from pond sediment. Bioresour. Technol. 2011, 102, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Ozmihci, S.; Kargi, F. Bio-hydrogen production by photo-fermentation of dark fermentation effluent with intermittent feeding and effluent removal. Int. J. Hydrog. Energy 2010, 35, 6674–6680. [Google Scholar] [CrossRef]
- Özkan, E.; Uyar, B.; Özgür, E.; Yücel, M.; Eroglu, I.; Gündüz, U. Photofermentative hydrogen production using dark fermentation effluent of sugar beet thick juice in outdoor conditions. Int. J. Hydrog. Energy 2012, 37, 2044–2049. [Google Scholar] [CrossRef]
- Venkateswar Reddy, M.; Venkata Mohan, S. Influence of aerobic and anoxic microenvironments on polyhydroxyalkanoates (PHA) production from food waste and acidogenic effluents using aerobic consortia. Bioresour. Technol. 2012, 103, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Cusick, R.D.; Kiely, P.D.; Logan, B.E. A monetary comparison of energy recovered from microbial fuel cells and microbial electrolysis cells fed winery or domestic wastewaters. Int. J. Hydrog. Energy 2010, 35, 8855–8861. [Google Scholar] [CrossRef]
- Wagner, R.C.; Regan, J.M.; Oh, S.-E.; Zuo, Y.; Logan, B.E. Hydrogen and methane production from swine wastewater using microbial electrolysis cells. Water Res. 2009, 43, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Goud, R.K.; Sarkar, O.; Chiranjeevi, P.; Venkata Mohan, S. Bioaugmentation of potent acidogenic isolates: A strategy for enhancing biohydrogen production at elevated organic load. Bioresour. Technol. 2014, 165, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Vijaya Bhaskar, Y.; Murali Krishna, P.; Chandrasekhara Rao, N.; Lalit Babu, V.; Sarma, P.N. Biohydrogen production from chemical wastewater as substrate by selectively enriched anaerobic mixed consortia: Influence of fermentation ph and substrate composition. Int. J. Hydrog. Energy 2007, 32, 2286–2295. [Google Scholar] [CrossRef]
- Marone, A.; Massini, G.; Patriarca, C.; Signorini, A.; Varrone, C.; Izzo, G. Hydrogen production from vegetable waste by bioaugmentation of indigenous fermentative communities. Int. J. Hydrog. Energy 2012, 37, 5612–5622. [Google Scholar] [CrossRef]
- Wang, A.; Ren, N.; Shi, Y.; Lee, D.-J. Bioaugmented hydrogen production from microcrystalline cellulose using co-culture—clostridium acetobutylicum and ethanoigenens harbinense. Int. J. Hydrog. Energy 2008, 33, 912–917. [Google Scholar] [CrossRef]
- Ma, F.; Guo, J.-B.; Zhao, L.-J.; Chang, C.-C.; Cui, D. Application of bioaugmentation to improve the activated sludge system into the contact oxidation system treating petrochemical wastewater. Bioresour. Technol. 2009, 100, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Falkentoft, C.; Venkata Nancharaiah, Y.; Sturm, B.S.M.; Wattiau, P.; Wilderer, P.A.; Wuertz, S.; Hausner, M. Bioaugmentation of microbial communities in laboratory and pilot scale sequencing batch biofilm reactors using the tol plasmid. Bioresour. Technol. 2009, 100, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Bouchez, T.; Patureau, D.; Dabert, P.; Wagner, M.; Delgenès, J.P.; Moletta, R. Successful and unsuccessful bioaugmentation experiments monitored by fluorescent in situ hybridization. Water Sci. Technol. 2000, 41, 61–68. [Google Scholar] [PubMed]
- Chandrasekhar, K.; Venkata Mohan, S. Bio-electrochemical remediation of real field petroleum sludge as an electron donor with simultaneous power generation facilitates biotransformation of pah: Effect of substrate concentration. Bioresour. Technol. 2012, 110, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.R.; Devi, M.P.; Reddy, M.; Chandrasekhar, K.; Juwarkar, A.; Sarma, P.N. Bioremediation of petroleum sludge under anaerobic microenvironment: Influence of biostimulation and bioaugmentation. Environ. Eng. Manag. J. 2011, 10, 1609–1616. [Google Scholar]
- Chong, M.-L.; Rahim, R.A.; Shirai, Y.; Hassan, M.A. Biohydrogen production by clostridium butyricum eb6 from palm oil mill effluent. Int. J. Hydrog. Energy 2009, 34, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.V.; Devi, M.P.; Chandrasekhar, K.; Goud, R.K.; Venkata Mohan, S. Aerobic remediation of petroleum sludge through soil supplementation: Microbial community analysis. J. Hazard. Mater. 2011, 197, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Chandrasekhar, K.; Venkata Mohan, S. Influence of carbohydrates and proteins concentration on fermentative hydrogen production using canteen based waste under acidophilic microenvironment. J. Biotechnol. 2011, 155, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.; Das, D. Hydrogen from biomass. Curr. Sci. 2003, 85, 265–271. [Google Scholar]
- Taherzadeh, M.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.-Q.; Guo, W.-Q.; Wang, X.-J.; Xiang, W.-S.; Liu, B.-F.; Wang, X.-Z.; Ding, J.; Chen, Z.-B. Effects of different pretreatment methods on fermentation types and dominant bacteria for hydrogen production. Int. J. Hydrog. Energy 2008, 33, 4318–4324. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Goud, R.K. Pretreatment of biocatalyst as viable option for sustained production of biohydrogen from wastewater treatment. In Biogas Production; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 291–311. [Google Scholar]
- Srikanth, S.; Venkata Mohan, S.; Lalit Babu, V.; Sarma, P.N. Metabolic shift and electron discharge pattern of anaerobic consortia as a function of pretreatment method applied during fermentative hydrogen production. Int. J. Hydrog. Energy 2010, 35, 10693–10700. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. Int. J. Mol. Sci. 2015, 16, 8266-8293. https://doi.org/10.3390/ijms16048266
Chandrasekhar K, Lee Y-J, Lee D-W. Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. International Journal of Molecular Sciences. 2015; 16(4):8266-8293. https://doi.org/10.3390/ijms16048266
Chicago/Turabian StyleChandrasekhar, Kuppam, Yong-Jik Lee, and Dong-Woo Lee. 2015. "Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes" International Journal of Molecular Sciences 16, no. 4: 8266-8293. https://doi.org/10.3390/ijms16048266
APA StyleChandrasekhar, K., Lee, Y. -J., & Lee, D. -W. (2015). Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. International Journal of Molecular Sciences, 16(4), 8266-8293. https://doi.org/10.3390/ijms16048266