New Hybrid Nanomaterial Based on Self-Assembly of Cyclodextrins and Cobalt Prussian Blue Analogue Nanocubes
Abstract
:1. Introduction
2. Results
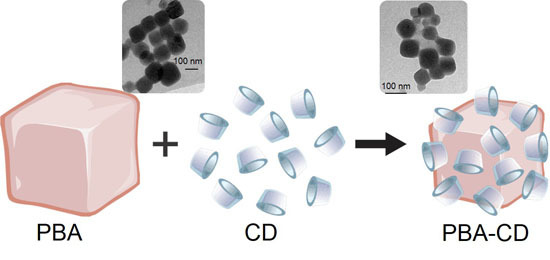
2.1. Supramolecular Arrangement

2.2. Spectroscopic Analyses


2.3. Structure and Crystallinity

2.4. Electrochemical Study

3. Discussion
3.1. Characterization of Self-Assembled Supramolecular Nanocubes

3.2. Electrochemical Characterization and Electron Transfer Study
4. Experimental Section
4.1. Chemicals and Materials
4.2. Synthesis of Co3[Co(CN)6]2 Nanocubes (Co3[Co(CN)6]2 NCs)
4.3. Synthesis of Co3[Co(CN)6]2 Nanocubes Decorated with β-Cyclodextrin (Co3[Co(CN)6]2-CD NCs)
4.4. Characterizations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tian, J.; Xu, J.; Zhu, F.; Lu, T.; Su, C.; Ouyang, G. Application of nanomaterials in sample preparation. J. Chromatogr. A 2013, 1300, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Barkalina, N.; Charalambous, C.; Jones, C.; Coward, K. Nanotechnology in reproductive medicine: Emerging applications of nanomaterials. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 921–938. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Grzybowski, B. Self-Assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Lopez, M.D.C.; Räisänen, M.T.; Chamberlain, T.W.; Weber, U.; Lebedeva, M.; Rance, G.A.; Briggs, G.A.D.; Pettifor, D.; Burlakov, V.; Buck, M.; et al. Functionalized fullerenes in self-assembled monolayers. Langmuir 2011, 27, 10977–10985. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Yun, Y.-H.; Rust, M.J.; Do, J.; Shanov, V.; Schulz, M.J.; Ahn, C.H. The precise self-assembly of individual carbon nanotubes using magnetic capturing and fluidic alignment. Nanotechnology 2009, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.R.; Paik, T.; Klein, D.R.; Naik, G.V.; Caglayan, H.; Boltasseva, A.; Murray, C.B. Shape-dependent plasmonic response and directed self-assembly in a new semiconductor building block, indium-doped cadmium oxide (ICO). Nano Lett. 2013, 13, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal-organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Baratella, D.; Salviulo, G.; Polakova, K.; Zoppellaro, G.; Tucek, J.; Kaslik, J.; Zboril, R.; Vianello, F. Core-shell hybrid nanomaterial based on prussian blue and surface active maghemite nanoparticles as stable electrocatalyst. Biosens. Bioelectron. 2014, 52, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.T.B.; Coelho, A.G.; Lopes, L.C.S.; Martins, M.V.A.; Crespilho, F.N.; Merkoçi, A.; Silva, W.C. Nano-assembled supramolecular films from chitosan-stabilized gold nanoparticles and Cobalt(II) phthalocyanine. J. Braz. Chem. Soc. 2013, 24, 1237–1245. [Google Scholar] [CrossRef]
- Leung, K.C.-F.; Xuan, S.; Zhu, X.; Wamg, D.; Chak, C.-P.; Lee, S.-F.; Ho, W.K.-W.; Chung, B.C.-T. Gold and iron oxide hybrid nanocomposite materials. Chem. Soc. Rev. 2012, 41, 1911–1928. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q.M. Magnetic nanocomposites with mesoporous structures: Synthesis and applications. Small 2011, 7, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Ciabocco, M.; Berrettoni, M.; Chillura, D.F.M.; Giorgetti, M. Electrochemistry of TiO2–iron hexacyanocobaltate composite electrodes. Solid State Ion. 2014, 259, 53–58. [Google Scholar] [CrossRef]
- Cao, M.; Wu, X.; He, X.; Hu, C. Shape-controlled synthesis of Prussian blue analogue Co3[Co(CN)6]2 nanocrystals. Chem. Commun. 2005, 17, 2241–2243. [Google Scholar] [CrossRef] [PubMed]
- Shriver, D.F.; Brown, D.B. The encironment of interstitial ions in a prussian blue analog, Co3[Co(CN)6]2. Inorg. Chem. 1969, 8, 42–46. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, P.; Chen, Q.-W.; Mei, J.-Y.; Yan, N. Room-temperature synthesis of Prussian blue analogue Co3[Co(CN)6]2 porous nanostructures and their CO2 storage properties. RSC Adv. 2011, 1, 1574–1578. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Culp, J.T.; Matranga, C.; Bockrath, B. Adsorption properties of hydrogen and carbon dioxide in Prussian blue analogues M3[Co(CN)6]2, M = Co, Zn. J. Phys. Chem. C 2007, 111, 1055–1060. [Google Scholar] [CrossRef]
- Nie, P.; Shen, L.; Luo, H.; Ding, B.; Xu, G.; Wang, J.; Zhang, X. Prussian blue analogues: A new class of anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 5852–5857. [Google Scholar] [CrossRef]
- Beauvais, L.G.; Long, J.R. Co3[Co(CN)5]2: A microporous magnet with an ordering temperature of 38 K. J. Am. Chem. Soc. 2002, 124, 12096–12097. [Google Scholar] [CrossRef] [PubMed]
- Buchold, D.H.M.; Feldmann, C. Synthesis of nanoscale in reverse microemulsions. Chem. Mater. 2007, 19, 3376–3380. [Google Scholar] [CrossRef]
- Ogoshi, T.; Hashizume, M.; Yamagishi, T.-A.; Nakamoto, Y. Chemically responsive supramolecular assemblies of pyrene-β-cyclodextrin dimer. Langmuir 2010, 26, 3169–3173. [Google Scholar] [CrossRef] [PubMed]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Astray, G.; Mejuto, J.C.; Morales, J.; Rial-Otero, R.; Simal-Gándara, J. Factors controlling flavors binding constants to cyclodextrins and their applications in foods. Food Res. Int. 2010, 43, 1212–1218. [Google Scholar] [CrossRef]
- Cid, A.; Mejuto, J.C.; Orellana, P.G.; López-Fernández, O.; Rial-Otero, R.; Simal-Gandara, J. Effects of ascorbic acid on the microstructure and properties of SDS micellar aggregates for potential food applications. Food Res. Int. 2013, 50, 143–148. [Google Scholar] [CrossRef]
- Cid, A.; Morales, J.; Mejuto, J.C.; Briz-Cid, N.; Rial-Otero, R.; Simal-Gandara, J. Thermodynamics of sodium dodecyl sulphate-salicylic acid based micellar systems and their potential use in fruits postharvest. Food Chem. 2014, 151, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, K.; Chen, Y.; Xi, F. Simple, clean preparation method for cross-linked α-cyclodextrin nanoparticles via inclusion complexation. Langmuir 2013, 29, 5939–5943. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.R.; Rolim, L.A.; Soares, M.F.L.R.; Rolim-Neto, P.J.; Albuquerque, M.M.; Soares-Sobrinho, J.L. Inclusion complex of methyl-β-cyclodextrin and olanzapine as potential drug delivery system for schizophrenia. Carbohydr. Polym. 2012, 89, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Li, J.; Luo, Z.; Hu, Y.; Hou, Y.; Ding, X. β-Cyclodextrin conjugated magnetic nanoparticles for diazepam removal from blood. Chem. Commun. 2011, 47, 7719–7721. [Google Scholar] [CrossRef] [PubMed]
- Bagabas, A.A.; Frasconi, M.; Iehl, J.; Hauser, B.; Farha, O.K.; Hupp, J.T.; Hartlieb, K.J.; Botros, Y.Y.; Stoddart, J.F. γ-Cyclodextrin cuprate sandwich-type complexes. Inorg. Chem. 2013, 52, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tang, Y.; Hu, W.; Tian, R.; Jia, Y.; Deng, P.; Zhang, L. Investigation of inclusion complex of honokiol with sunfobutyl ether-β-cyclodextrin. Carbohydr. Polym. 2014, 113, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, J.-B.; Xiao, C.-F.; Liu, Z.-C.; Gao, Z.-Y.; Yan, S.-J.; Zhang, J.-H.; Zhang, H.-B.; Lin, J. Inclusion complex of GA-13316 with β-cyclodextrin: Preparation, characterization, molecular modeling, and in vitro evaluation. Carbohydr. Polym. 2014, 111, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, R.; Rajamohan, R.; Kothainayaki, S.; Sivakumar, K. Spectral investigation and structural characterization of dibenzalacetone: β-Cyclodextrin inclusion complex. J. Mol. Struct. 2014, 1068, 155–163. [Google Scholar] [CrossRef]
- Srinivasan, K.; Sivakumar, K.; Stalin, T. 2,6-dinitroaniline and β-cyclodextrin inclusion complex properties studied by different analytical methods. Carbohydr. Polym. 2014, 133, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.C.; Guix, M.; Angeles, G.A.; Merkoçi, A. Compact microcubic structures platform based on self-assembly Prussian blue nanoparticles with highly tuneable conductivity. Phys. Chem. Chem. Phys. 2010, 12, 15505–15511. [Google Scholar] [CrossRef] [PubMed]
- Pyrasch, M.; Toutianoush, A.; Jin, W.; Schnepf, J.; Tieke, B. Self-assembled films of Prussian blue and analogues: Optical and electrochemical properties and application as ion-sieving membranes. Chem. Mater. 2003, 15, 245–254. [Google Scholar] [CrossRef]
- Miskowski, V.M.; Gray, H.B.; Wilson, R.B.; Solomon, E.I. Position of the 3T1g ← 1A1g transition in hexacyanocobaltate(III). Analysis of absorption and emission results. Inorg. Chem. 1979, 18, 1410–1412. [Google Scholar] [CrossRef]
- Bitmez, S.; Sayin, K.; Avar, B.; Köse, M.; Kayraldiz, A.; Kurtoglu, M. Preparation, spectral, X-ray powder diffraction and computational studies and genotoxic properties of new azo-azomethine metal chelates. J. Mol. Struct. 2014, 1076, 213–226. [Google Scholar] [CrossRef]
- Sambasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.A. Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Eckermann, A.L.; Feld, D.J.; Shaw, J.A.; Meade, T.J. Electrochemistry of redox-active self-assembled monolayers. Coord. Chem. Rev. 2010, 254, 1769–1802. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.A. On the theory of oxidation-reduction reactions involving electron transfer. J. Chem. Phys. 1956, 24, 966–978. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophy. Acta 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Jouni, Z.E.; Zamora, J.; Snyder, M.; Montfort, W.R.; Weichsel, A.; Wells, M.A. α-Cyclodextrin extracts diacylglycerol from insect high density lipoproteins. J. Lipid Res. 2000, 41, 933–939. [Google Scholar] [PubMed]
- Melo, A.F.A.A.; Luz, R.A.S.; Iost, R.M.; Nantes, I.L.; Crespilho, F.N. Highly stable magnetite modified with chitosan, ferrocene and enzyme for application in magneto-switchable bioelectrocatalysis. J. Braz. Chem. Soc. 2013, 24, 285–294. [Google Scholar] [CrossRef]
- Lejeune, J.; Brubach, J.-B.; Roy, P.; Bleuzen, A. Application of the infrared spectroscopy to the structural study of Prussian blue analogues. Comptes Rendus Chim. 2014, 17, 534–540. [Google Scholar] [CrossRef]
- Nazi, M.; Malek, R.M.A.; Kotek, R. Modification of β-cyclodextrin with itaconic acid and application of the new derivative to cotton fabrics. Carbohydr. Polym. 2012, 88, 950–958. [Google Scholar] [CrossRef]
- Yan, N.; Hu, L.; Li, Y.; Whang, Y.; Zhong, H.; Hu, X.; Kong, X.; Chen, Q. Co3O4 Nanocages for high-performance anode material in lithium-ion batteries. J. Phys. Chem. C 2012, 116, 7227–7235. [Google Scholar] [CrossRef]
- Nikolic, V.; Stankovic, M.; Kapor, A.; Nikolic, L.; Cvetkovic, D.; Stamenkovic, J. Allylthiosulfinate: β-cyclodextrin inclusion complex: Preparation, characterization and microbiological activity. Pharmazie 2004, 59, 845–848. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, C.L.C.; Silva, A.T.B.; Macedo, L.J.A.; Luz, R.A.S.; Neto, J.M.M.; Filho, U.P.R.; Cantanhêde, W. New Hybrid Nanomaterial Based on Self-Assembly of Cyclodextrins and Cobalt Prussian Blue Analogue Nanocubes. Int. J. Mol. Sci. 2015, 16, 14594-14607. https://doi.org/10.3390/ijms160714594
Carvalho CLC, Silva ATB, Macedo LJA, Luz RAS, Neto JMM, Filho UPR, Cantanhêde W. New Hybrid Nanomaterial Based on Self-Assembly of Cyclodextrins and Cobalt Prussian Blue Analogue Nanocubes. International Journal of Molecular Sciences. 2015; 16(7):14594-14607. https://doi.org/10.3390/ijms160714594
Chicago/Turabian StyleCarvalho, Caio L. C., Anna T. B. Silva, Lucyano J. A. Macedo, Roberto A. S. Luz, José M. Moita Neto, Ubirajara P. Rodrigues Filho, and Welter Cantanhêde. 2015. "New Hybrid Nanomaterial Based on Self-Assembly of Cyclodextrins and Cobalt Prussian Blue Analogue Nanocubes" International Journal of Molecular Sciences 16, no. 7: 14594-14607. https://doi.org/10.3390/ijms160714594
APA StyleCarvalho, C. L. C., Silva, A. T. B., Macedo, L. J. A., Luz, R. A. S., Neto, J. M. M., Filho, U. P. R., & Cantanhêde, W. (2015). New Hybrid Nanomaterial Based on Self-Assembly of Cyclodextrins and Cobalt Prussian Blue Analogue Nanocubes. International Journal of Molecular Sciences, 16(7), 14594-14607. https://doi.org/10.3390/ijms160714594