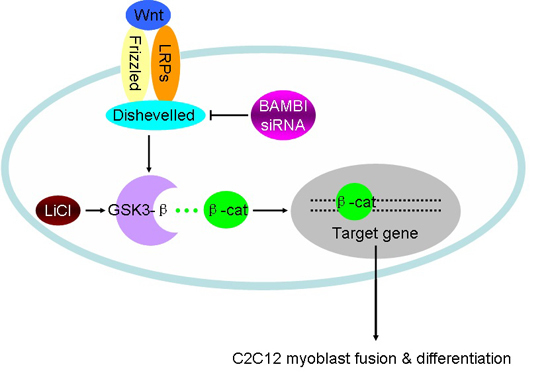
BAMBI Promotes C2C12 Myogenic Differentiation by Enhancing Wnt/β-Catenin Signaling
Abstract
:1. Introduction
2. Results
2.1. Expression Patterns of BAMBI

2.2. Knockdown of BAMBI Inhibits C2C12 Myogenic Differentiation
2.3. Knockdown of BAMBI Inhibits Wnt/β-Catenin Signaling during C2C12 Differentiation


2.4. LiCl Rescued the Influence of BAMBI Interference on C2C12 Differentiation

3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. Transfection of BAMBI siRNA and the siRNA Negative Control
| siRNA | Sequences (5′–3′) | |
|---|---|---|
| BAMBI siRNA | Sense | GCAAGCAGAGCUCAGUAAUTT |
| Antisense | AUUACUGAGCUCUGCUUGCTT | |
| Negative control | Sense | GCAAGCAGAGCUCAGUAAUTT |
| Antisense | ACGUGACACGUUCGGAGAATT | |
4.3. Real-Time Quantitative PCR
4.4. Western Blot Detection
| Gene | Sequences (5′–3′) | Accession No. |
|---|---|---|
| MyoD | F: CATTCCAACCCACAGAACCT | NM_010866.1 |
| R: CAAGCCCTGAGAGTCGTCTT | ||
| MyoG | F: CAATGCACTGGAGTTCGGT | NM_031189.2 |
| R: CTGGGAAGGCAACAGACAT | ||
| MyHC | F: CGCAAGAATGTTCTCAGGCT | NM_030679.1 |
| R: GCCAGGTTGACATTGGATTG | ||
| Axin2 | F: CGCTCGGGTTTGTGTTAAGT | NM_015732.4 |
| R: GTCAACGCTCTGCCCTACAC | ||
| BAMBI | F: AAGCCTCAGGACAAGGAAA | NM_026505.2 |
| R: CAATGGGAACCGCTATCA | ||
| GAPDH | F: AACTTTGGCATTGTGGAAGG | NM_008084.3 |
| R: ACACATTGGGGGTAGGAACA |
4.5. Immunocytochemical Analysis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Onichtchouk, D.; Chen, Y.G.; Dosch, R.; Gawantka, V.; Delius, H.; Massague, J.; Niehrs, C. Silencing of TGF-β signalling by the pseudoreceptor BAMBI. Nature 1999, 401, 480–485. [Google Scholar] [PubMed]
- Yan, X.; Lin, Z.; Chen, F.; Zhao, X.; Chen, H.; Ning, Y.; Chen, Y.G. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-β signaling. J. Biol. Chem. 2009, 284, 30097–30104. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Song, X.; Ma, H.; Liu, L.; Wen, X.; Yu, J.; Wang, L.; Hu, S. Knockdown of BAMBI inhibits β-catenin and transforming growth factor β to suppress metastasis of gastric cancer cells. Mol. Med. Rep. 2014, 10, 874–880. [Google Scholar] [PubMed]
- Zhou, L.; Park, J.; Jang, K.Y.; Park, H.S.; Wagle, S.; Yang, K.H.; Lee, K.; Park, B.; Kim, J.R. The overexpression of BAMBI and its involvement in the growth and invasion of human osteosarcoma cells. Oncol. Rep. 2013, 30, 1315–1322. [Google Scholar] [PubMed]
- Mai, Y.; Zhang, Z.; Yang, H.; Dong, P.; Chu, G.; Yang, G.; Sun, S. BMP and activin membrane-bound inhibitor (BAMBI) inhibits the adipogenesis of porcine preadipocytes through Wnt/β-catenin signaling pathway. Biochem. Cell Biol. 2014, 92, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hutley, L.J.; Webster, J.A.; Kim, Y.H.; Liu, D.F.; Newell, F.S.; Widberg, C.H.; Bachmann, A.; Turner, N.; Schmitz-Peiffer, C.; et al. Identification of BMP and activin membrane-bound inhibitor (BAMBI) as a potent negative regulator of adipogenesis and modulator of autocrine/paracrine adipogenic factors. Diabetes 2012, 61, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, L.; Ti, X.; Krause, U.; Hai, B.; Zhao, Y.; Yang, Z.; Liu, F. Inhibition of TGF-β/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells 2012, 30, 2810–2819. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Gao, C.; Ning, Y.; He, X.; Wu, W.; Chen, Y.G. The pseudoreceptor BMP and activin membrane-bound inhibitor positively modulates Wnt/β-catenin signaling. J. Biol. Chem. 2008, 283, 33053–33058. [Google Scholar] [CrossRef] [PubMed]
- Steinman, E.D.; Mattison, G.L.; Pira, C.U.; Oberg, K.C. Defining the mechanism of limb regeneration: A potential novel role for BAMBI in mediating Fgf-induced Shh up-regulation. In The FASEB Journal; In Proceedings of the Experimental Biology Meeting 2011, Washington, DC, USA, 9–13 April 2011; Federation of American Societies for Experimental Biology: Bethesda, MD, USA, 2011. [Google Scholar]
- Brack, A.S.; Conboy, I.M.; Conboy, M.J.; Shen, J.; Rando, T.A. A temporal switch from Notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2008, 2, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Jin, Y.; Tan, L.; Kosciuk, T.; Lee, J.; Yoon, J.K. Regulation of the Follistatin gene by RSPO-LGR4 signaling via activation of the Wnt/β-catenin pathway in skeletal myogenesis. Mol. Cell. Biol. 2014, 34, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Han, X.H.; Jin, Y.R.; Seto, M.; Yoon, J.K. A WNT/β-catenin signaling activator, R-spondin, plays positive regulatory roles during skeletal myogenesis. J. Biol. Chem. 2011, 286, 10649–10659. [Google Scholar] [CrossRef] [PubMed]
- Rochat, A.; Fernandez, A.; Vandromme, M.; Moles, J.P.; Bouschet, T.; Carnac, G.; Lamb, N. Insulin and Wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol. Biol. Cell 2004, 15, 4544–4555. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Fang, Y.H.; Chi, H.C.; Chang, L.C.; Chung, S.Y.; Huang, W.C.; Wang, X.W.; Lee, K.W.; Chen, S.L. Insulin and LiCl synergistically rescue myogenic differentiation of FoxO1 over-expressed myoblasts. PLoS ONE 2014, 9, e88450. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building Muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Von Maltzahn, J.; Chang, N.C.; Bentzinger, C.F.; Rudnicki, M.A. Wnt signaling in myogenesis. Trends Cell Biol. 2012, 22, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Ridgeway, A.G.; Petropoulos, H.; Wilton, S.; Skerjanc, I.S. Wnt signaling regulates the function of MyoD and myogenin. J. Biol. Chem. 2000, 275, 32398–32405. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, S.; Hidaka, R.; Asashima, M.; Takemasa, T.; Kuwabara, T. Wnt protein-mediated satellite cell conversion in adult and aged mice following voluntary wheel running. J. Biol. Chem. 2014, 289, 7399–7412. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Neiswender, H.; Baik, E.J.; Xiong, W.C.; Mei, L. β-catenin interacts with MyoD and regulates its transcription activity. Mol. Cell. Biol. 2008, 28, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Zhang, Z.; Dong, P.; Yang, H.; Yang, G.; Sun, S. BAMBI inhibits porcine preadipocyte differentiation by facilitating ERK1/2 phosphorylation. Sheng Wu Gong Cheng Xue Bao 2014, 30, 1531–1540. (In Chinese) [Google Scholar] [PubMed]
- Jho, E.H.; Zhang, T.; Domon, C.; Joo, C.K.; Freund, J.N.; Costantini, F. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002, 22, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.Y. Activation of axin2 expression by β-catenin-T Cell Factor. A feedback repressor pathway regulating Wnt signaling. J. Biol. Chem. 2002, 277, 21657–21665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, S.; Zhang, J.; Zhou, F.; Ten, D.P. Wnt/β-catenin signaling changes C2C12 myoblast proliferation and differentiation by inducing Id3 expression. Biochem. Biophys. Res. Commun. 2012, 419, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.S.; Melton, D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA. 1996, 93, 8455–8459. [Google Scholar] [CrossRef] [PubMed]
- Hedgepeth, C.M.; Conrad, L.J.; Zhang, J.; Huang, H.; Lee, V.M.Y.; Klein, P.S. Activation of the Wnt signaling pathway: A molecular mechanism for lithium action. Dev. Biol. 1997, 185, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Adachi, S.; Kohu, K.; Yamada, T.; Higuchi, O.; Furukawa, Y.; Nakamura, Y.; Nakamura, T.; Tashiro, K.; Kuhara, S.; et al. Identification of BMP and activin membrane-bound inhibitor (BAMBI), an inhibitor of transforming growth factor-β signaling, as a target of the β-catenin pathway in colorectal tumor cells. J. Biol. Chem. 2004, 279, 6840–6846. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mo, C.; Romero-Suarez, S.; Bonewald, L.; Brotto, M. Wnt3a potentiates myogenesis in C2C12 myoblasts by orchestrated changes in IP3-mediated Calcium signaling and β-catenin activation. In Proceedings of Annual Meeting of the American-Society-for-Bone-and-Mineral-Research 2013, Baltimore, MD, USA, 4–7 October 2013; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Langen, R.; van der Velden, J.; Kelders, M.; Laeremans, H.; Schols, A. Wnt3a promotes β-catenin signaling and myotube formation during myogenic differentiation. FASEB J. 2008, 22, 754.21. [Google Scholar]
- Yaffe, D.; Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Shi, X.-E.; Song, C.; Sun, S.; Yang, G.; Li, X. BAMBI Promotes C2C12 Myogenic Differentiation by Enhancing Wnt/β-Catenin Signaling. Int. J. Mol. Sci. 2015, 16, 17734-17745. https://doi.org/10.3390/ijms160817734
Zhang Q, Shi X-E, Song C, Sun S, Yang G, Li X. BAMBI Promotes C2C12 Myogenic Differentiation by Enhancing Wnt/β-Catenin Signaling. International Journal of Molecular Sciences. 2015; 16(8):17734-17745. https://doi.org/10.3390/ijms160817734
Chicago/Turabian StyleZhang, Qiangling, Xin-E Shi, Chengchuang Song, Shiduo Sun, Gongshe Yang, and Xiao Li. 2015. "BAMBI Promotes C2C12 Myogenic Differentiation by Enhancing Wnt/β-Catenin Signaling" International Journal of Molecular Sciences 16, no. 8: 17734-17745. https://doi.org/10.3390/ijms160817734
APA StyleZhang, Q., Shi, X. -E., Song, C., Sun, S., Yang, G., & Li, X. (2015). BAMBI Promotes C2C12 Myogenic Differentiation by Enhancing Wnt/β-Catenin Signaling. International Journal of Molecular Sciences, 16(8), 17734-17745. https://doi.org/10.3390/ijms160817734