Possible Immune Regulation of Natural Killer T Cells in a Murine Model of Metal Ion-Induced Allergic Contact Dermatitis
Abstract
:1. Introduction
2. Establishment of Murine Models for Metal Allergy
3. Immune Responses in Inflamed Metal ACD in Terms of the TCR
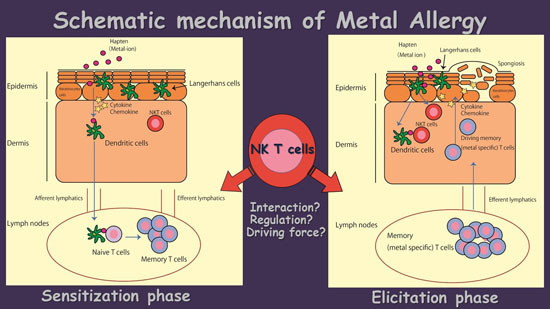
4. The Possible Role of NK T Cells in Metal Allergy

5. Conclusions and Future Direction
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raap, U.; Stiesch, M.; Reh, H.; Kapp, A.; Werfel, T. Investigation of contact allergy to dental metals in 206 patients. Contact Dermatitis 2009, 60, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kalish, R.S. Recent developments in the pathogenesis of allergic contact dermatitis. Arch. Dermatol. 1991, 127, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Kinbara, M.; Kuroishi, T.; Kimura, K.; Iwakura, Y.; Ohtsu, H.; Sugawara, S.; Endo, Y. Lipopolysaccharide promotes and augments metal allergies in mice, dependent on innate immunity and histidine decarboxylase. Clin. Exp. Allergy 2007, 37, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Kinbara, M.; Sato, N.; Kuroishi, T.; Takano-Yamamoto, T.; Sugawara, S.; Endo, Y. Allergy-inducing nickel concentration is lowered by lipopolysaccharide at both the sensitization and elicitation steps in a murine model. Br. J. Dermatol. 2011, 164, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, N.A.; Kronenberg, M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 2007, 178, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.D.; Li, Q.; Liu, J.; McClellan, S.; Du, W.; Barrett, R.P. NKT cells are critical to initiate an inflammatory response after Pseudomonas aeruginosa ocular infection in susceptible mice. J. Immunol. 2007, 179, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Raghavan, B.; Müller, V.; Vogl, T.; Fejer, G.; Tchaptchet, S.; Keck, S.; Kalis, C.; Nielsen, P.J.; Galanos, C.; et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat. Immunol. 2010, 11, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kumagai, K.; Eguchi, T.; Shigematsu, H.; Kitaura, K.; Kawano, M.; Horikawa, T.; Suzuki, S.; Matsutani, T.; Ogasawara, K.; et al. Characterization of T cell receptors of Th1 cells infiltrating inflamed skin of a novel murine model of palladium-induced metal allergy. PLoS ONE 2013, 8, e76385. [Google Scholar]
- Eguchi, T.; Kumagai, K.; Kobayashi, H.; Shigematsu, H.; Kitaura, K.; Suzuki, S.; Horikawa, T.; Hamada, Y.; Ogasawara, K.; Suzuki, R. Accumulation of invariant NKT cells into inflamed skin in a novel murine model of nickel allergy. Cell Immunol. 2013, 284, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, H.; Kumagai, K.; Kobayashi, H.; Eguchi, T.; Kitaura, K.; Suzuki, S.; Horikawa, T.; Matsutani, T.; Ogasawara, K.; Hamada, Y.; et al. Accumulation of metal-specific T cells in inflamed skin in a novel murine model of chromium-induced allergic contact dermatitis. PLoS ONE 2014, 9, e85983. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Goi, Y.; Hong, J.J.; Seyama, T.; Ohtsu, H.; Wada, H.; Ohuchi, K.; Hirasawa, N. Effects of nickel on eosinophil survival. Int. Arch. Allergy Immunol. 2009, 149, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Huh, K.; Lee, K.Y.; Yang, J.M.; Kim, T.J. Nickel induces secretion of IFN-gamma by splenic natural killer cells. Exp. Mol. Med. 2009, 41, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Matsutani, T.; Yoshioka, T.; Tsuruta, Y.; Iwagami, S.; Suzuki, R. Analysis of TCRAV and TCRBV repertoires in healthy individuals by microplate hybridization assay. Hum. Immunol. 1997, 56, 57–69. [Google Scholar] [CrossRef]
- Gotoh, A.; Hamada, Y.; Shiobara, N.; Kumagai, K.; Seto, K.; Horikawa, T.; Suzuki, R. Skew in T cell receptor usage with polyclonal expansion in lesions of oral lichen planus without hepatitis C virus infection. Clin. Exp. Immunol. 2008, 154, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Hamada, Y.; Gotoh, A.; Kobayashi, H.; Kawaguchi, K.; Horie, A.; Yamada, H.; Suzuki, S.; Suzuki, R. Evidence for the changes of antitumor immune response during lymph node metastasis in head and neck squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, K.; Fujii, Y.; Hayasaka, D.; Matsutani, T.; Shirai, K.; Nagata, N.; Lim, C.K.; Suzuki, S.; Takasaki, T.; Suzuki, R.; et al. High clonality of virus-specific T lymphocytes defined by TCR usage in the brains of mice infected with West Nile virus. J. Immunol. 2011, 15, 3919–3930. [Google Scholar] [CrossRef] [PubMed]
- Engel, I.; Hedrick, SM. Site-directed mutations in the VDJ junctional region of a T cell receptor beta chain cause changes in antigenic peptide recognition. Cell 1988, 12, 473–484. [Google Scholar] [CrossRef]
- Davis, M.M.; Bjorkman, P.J. T-Cell antigen receptor genes and T cell recognition. Nature 1988, 4, 334–395. [Google Scholar]
- Puisieux, I.; Even, J.; Pannetier, C.; Jotereau, F.; Favrot, M.; Kourilsky, P. Oligoclonality of tumor-infiltrating lymphocytes from human melanomas. J. Immunol. 1994, 153, 2807–2818. [Google Scholar] [PubMed]
- Hashizume, H.; Seo, N.; Ito, T.; Takigawa, M.; Yagi, H. Promiscuous interaction between gold-specific T cells and APCs in gold allergy. J. Immunol. 2008, 181, 8096–8102. [Google Scholar] [CrossRef] [PubMed]
- Budinger, L.; Neuser, N.; Totzke, U.; Merk, H.F.; Hertl, M. Preferential usage of TCR-Vbeta17 by peripheral and cutaneous T cells in nickel-induced contact dermatitis. J. Immunol. 2001, 167, 6038–6044. [Google Scholar] [CrossRef] [PubMed]
- Silvennoinen-Kassinen, S.; Karvonen, J.; Ikaheimo, I. Restricted and individual usage of T cell receptor β-gene variables in nickel-induced CD4+ and CD8+ cells. Scand. J. Immunol. 1998, 48, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Harada, M.; Kojo, S.; Nakayama, T.; Wakao, H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 2003, 21, 483–513. [Google Scholar] [CrossRef] [PubMed]
- Sieling, P.A. CD1-Restricted T cells: T cells with a unique immunological niche. Clin. Immunol. 2000, 96, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Rivera, M.N.; Park, S.H.; Roark, J.H. Mouse CD1-specific NK1 T cells: Development, specificity, and function. Annu. Rev. Immunol. 1997, 15, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, B.J.; Kruisbeek, A.M.; Ton-That, H.; Weston, M.A.; Coligan, J.E.; Schwartz, R.H.; Pardoll, D.M. A novel population of T-cell receptor alpha betabearing thymocytes which predominantly expresses a single V β gene family. Nature 1987, 329, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Ceredig, R.; Lynch, F.; Newman, P. Phenotypic properties, interleukin 2 production, and developmental origin of a “mature” subpopulation of Lyt-2L3T4-mouse thymocytes. Proc. Natl. Acad. Sci. USA 1987, 84, 8578–8582. [Google Scholar] [CrossRef] [PubMed]
- Gober, M.D.; Fishelevich, R.; Zhao, Y.; Unutmaz, D.; Gaspari, A.A. Human natural killer T cells infiltrate into the skin at elicitation sites of allergic contact dermatitis. J. Investig. Dermatol. 2008, 128, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Costa, M. Transformation of human osteoblasts to anchorage-independent growth by insoluble nickel particles. Environ. Health Perspect. 1994, 102, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Shimizuhira, C.; Otsuka, A.; Honda, T. Natural killer T cells are essential for the development of contact hypersensitivity in BALB/c mice. J. Investig. Dermatol. 2014, 134, 2709–2718. [Google Scholar] [CrossRef] [PubMed]
- Kinbara, M.; Nagai, Y.; Takano-Yamamoto, T.; Sugawara, S.; Endo, Y. Cross-reactivity among some metals in a murine metal allergy model. Br. J. Dermatol. 2011, 165, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Almogren, A.; Adam, M.H.; Shakoor, Z.; Gadelrab, M.O.; Musa, H.A. Th1 and Th2 cytokine profile of CD4 and CD8 positive peripheral blood lymphocytes in nickel contact dermatitis. Cent. Eur. J. Immunol. 2013, 38, 100–106. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumagai, K.; Horikawa, T.; Shigematsu, H.; Matsubara, R.; Kitaura, K.; Eguchi, T.; Kobayashi, H.; Nakasone, Y.; Sato, K.; Yamada, H.; et al. Possible Immune Regulation of Natural Killer T Cells in a Murine Model of Metal Ion-Induced Allergic Contact Dermatitis. Int. J. Mol. Sci. 2016, 17, 87. https://doi.org/10.3390/ijms17010087
Kumagai K, Horikawa T, Shigematsu H, Matsubara R, Kitaura K, Eguchi T, Kobayashi H, Nakasone Y, Sato K, Yamada H, et al. Possible Immune Regulation of Natural Killer T Cells in a Murine Model of Metal Ion-Induced Allergic Contact Dermatitis. International Journal of Molecular Sciences. 2016; 17(1):87. https://doi.org/10.3390/ijms17010087
Chicago/Turabian StyleKumagai, Kenichi, Tatsuya Horikawa, Hiroaki Shigematsu, Ryota Matsubara, Kazutaka Kitaura, Takanori Eguchi, Hiroshi Kobayashi, Yasunari Nakasone, Koichiro Sato, Hiroyuki Yamada, and et al. 2016. "Possible Immune Regulation of Natural Killer T Cells in a Murine Model of Metal Ion-Induced Allergic Contact Dermatitis" International Journal of Molecular Sciences 17, no. 1: 87. https://doi.org/10.3390/ijms17010087
APA StyleKumagai, K., Horikawa, T., Shigematsu, H., Matsubara, R., Kitaura, K., Eguchi, T., Kobayashi, H., Nakasone, Y., Sato, K., Yamada, H., Suzuki, S., Hamada, Y., & Suzuki, R. (2016). Possible Immune Regulation of Natural Killer T Cells in a Murine Model of Metal Ion-Induced Allergic Contact Dermatitis. International Journal of Molecular Sciences, 17(1), 87. https://doi.org/10.3390/ijms17010087