Function and Regulation of Heterotrimeric G Proteins during Chemotaxis
Abstract
:1. Introduction
2. Regulation of GPCRs and Heterotrimeric G Proteins during Chemotaxis
2.1. Chemotaxis Receptors and Their Regulation
| Receptor | Ligand(s) | Cellular Expression |
|---|---|---|
| CCR5 | CCL2/3/4/5/13/15 | T cell, NK cell, monocyte, macrophage, dendritic cell |
| CCR6 | CCL19, β-defensin | B cell, T cell, NK cell, dendritic cell |
| CXCR2 | CCL28, CXCL1/2/5/6/7/8 | T cell, NK cell, neutrophil, monocyte, dendritic cell, granulocyte |
| CXCR4 | CXCL12 (SDF-1) | B cell, T cell, NK cell neutrophil, monocyte, macrophage, dendritic cell, granulocyte, neurons |
| CXCR5 | CXCL13 | B cell, T cell |
| BLT1/2 | LTB4 | B cell, T cell, neutrophil, monocyte, macrophage, dendritic cell, granulocyte |
| LPA1 | LPA | NK cell, macrophage |
| PAFR | PAF | B cell, neutrophil, monocyte |
| FPR1/2 | Formyl peptides | T cell, neutrophil, monocyte, macrophage, dendritic cell |
| A1 receptor | Adenosine | Neutrophil, monocyte, macrophage, dendritic cell |
| A2A receptor | Adenosine | B cell, NK cell, neutrophil, monocyte, macrophage, dendritic cell |
| A2B receptor | Adenosine | B cell, NK cell, neutrophil, monocyte, macrophage, dendritic cell |
| A3 receptor | Adenosine | B cell, NK cell, neutrophil, monocyte, macrophage, dendritic cell |
| cAR1 | cAMP | Dictyostelium. Peaks at 4 h of development, then drops dramatically, early aggregation |
| cAR2 | cAMP | Dictyostelium. Peaks at 16 h of development, mound formation |
| cAR3 | cAMP | Dictyostelium. Peaks at 4 h of development, then slowly decreases, late aggregation stage |
| cAR4 | cAMP | Dictyostelium. Peaks at 20 h of development, culmination |
| To be identified | Folic acid | Dictyostelium. Vegetative cells |
2.1.1. Ligand Binding Properties and Expression
2.1.2. Receptor Adaptation and Internalization

2.2. Kinetics and Regulation of Heterotrimeric G Proteins during Chemotaxis

2.2.1. Regulation of Gα Signaling by GEFs
| Classification | G Protein Selectivity | Chemotactic Downstream Pathway |
|---|---|---|
| GEF | ||
| GIV | Gαi3 | PI3K/Akt pathway |
| Mammalian Ric-8A | Gαi/o, Gαq, and Gα12 | Gαq-linked ERK activation |
| Mammalian Ric-8B | Gαs and Gαq | Not defined |
| D. discoideum Ric8 | Gα2and Gα4 | Ras, small G proteins |
| RGS | ||
| Mammalian RGS1 | Gαi | Down-regulation of Gβγ |
| Mammalian RGS3 | Gαi | Blocking binding of Gα to adenylyl cyclase |
| Mammalian RGS4 | Gαi | MAPK pathways: ERK1/2 and p38MAPKs |
| Mammalian RGS13 | Gαi and Gαq | Intracellular calcium production and pERK1/2 induction |
| D. discoideum RCK1 | Gα2 | Not defined |
| GDI | ||
| Mammalian AGS3/LGN | Gαi | Binding to Gαi-GDP and mInsc |
| Mammalian Rap1GAP | Gαz | Rap1/B-Raf/ERK pathway |
2.2.2. Regulation of Gα Signaling by RGS
2.2.3. Regulation of Gα Signaling by GDIs
2.2.4. Regulation of Gβγ Signaling
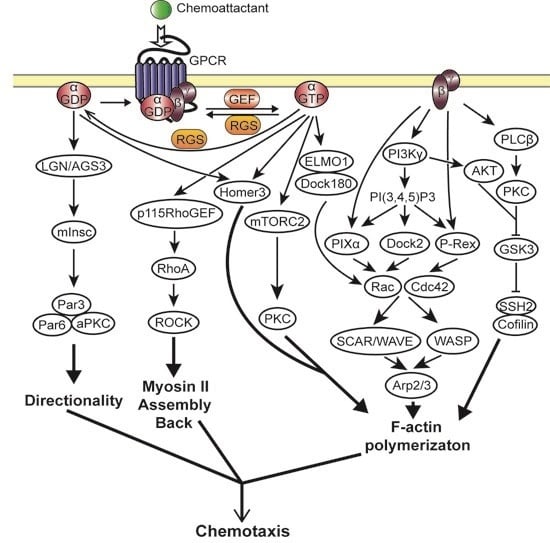
2.3. Heterotrimeric G Protein Activated Chemotaxis Pathways

2.3.1. Gβγ Mediated Chemotaxis Pathways
2.3.2. Gα Mediated Chemotaxis Pathways
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jin, T. Gradient sensing during chemotaxis. Curr. Opin. Cell Biol. 2013, 25, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Rott, A.; Butcher, E.C. Leukocyte chemoattractant receptors in human disease pathogenesis. Annu. Rev. Pathol. 2015, 10, 51–81. [Google Scholar] [CrossRef] [PubMed]
- Artemenko, Y.; Lampert, T.J.; Devreotes, P.N. Moving towards a paradigm: Common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes. Cell. Mol. Life Sci. 2014, 71, 3711–3747. [Google Scholar] [CrossRef] [PubMed]
- Bagorda, A.; Mihaylov, V.A.; Parent, C.A. Chemotaxis: Moving forward and holding on to the past. Thromb. Haemost. 2006, 95, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.M.; Veltman, D.; Kay, R.R. Chemotaxis of a model organism: Progress with Dictyostelium. Curr. Opin. Cell Biol. 2015, 36, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.M.; Losada, A.; Nanjundiah, V.; Konijn, T.M. Signal input for a chemotactic response in the cellular slime mold Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 1975, 72, 4991–4993. [Google Scholar] [CrossRef] [PubMed]
- Devreotes, P.N.; Potel, M.J.; MacKay, S. A Quantitative analysis of cyclic AMP waves mediating aggregation in Dictyostelium discoideum. Dev. Biol. 1983, 96, 405–415. [Google Scholar] [CrossRef]
- Oldham, W.M.; Hamm, H.E. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2008, 9, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Devreotes, P.N.; Zigmond, S.H. Chemotaxis in Eukaryotic Cells—A Focus on Leukocytes and Dictyostelium. Annu. Rev. Cell Biol. 1988, 4, 649–686. [Google Scholar] [CrossRef] [PubMed]
- Gether, U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 2000, 21, 90–113. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Tate, C.G.; Schertler, G.F.; Babu, M.M. Molecular signatures of G-protein-coupled receptors. Nature 2013, 494, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Saxe, C.L.; Johnson, R.; Devreotes, P.N.; Kimmel, A.R. Multiple genes for cell surface cAMP receptors in Dictyostelium discoideum. Dev. Genet. 1991, 12, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.-L.; Lijima, M. Signaling Mechanisms for Chemotaxis. Dev. Growth Differ. 2011, 53, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Nadkarni, S.M.; Bödeker, H.U.; Beta, C.; Bae, A.; Franck, C.; Rappel, W.-J.; Loomis, W.F.; Bodenschatz, E. Dictyostelium discoideum chemotaxis: Threshold for directed motion. Eur. J. Cell Biol. 2006, 85, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Devreotes, P.N.; Borleis, J.; Hereld, D. Agonist-induced loss of ligand binding is correlated with phosphorylation of cAR1, a G protein-coupled chemoattractant receptor from Dictyostelium. J. Biol. Chem. 1995, 270, 8667–8672. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Borleis, J.A.; Devreotes, P.N. Switching of chemoattractant receptors programs development and morphogenesis in Dictyostelium: Receptor subtypes activate common responses at different agonist concentrations. Dev. Biol. 1998, 197, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Barletta, K.E.; Ley, K.; Mehrad, B. Regulation of neutrophil function by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Junger, W.G. Immune cell regulation by autocrine purinergic signaling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Richardson, R.M.; Haribabu, B.; Snyderman, R. Chemoattractant receptor cross-desensitization. J. Biol. Chem. 1999, 274, 6027–6030. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Channon, K.M.; McNeill, E. The downstream regulation of chemokine receptor signalling: Implications for atherosclerosis. Mediators Inflamm. 2013, 2013, 459520. [Google Scholar] [CrossRef] [PubMed]
- Chidiac, P. RGS proteins destroy spare receptors: Effects of GPCR-interacting proteins and signal deamplification on measurements of GPCR agonist potency. Methods 2016, 92, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Laporte, S.A.; Miller, W.E.; Kim, K.-M.; Caron, M.G. β-Arrestin/AP-2 Interaction in G Protein-coupled Receptor Internalization: IDENTIFICATION OF A β-ARRESTIN BINDING SITE IN β2-ADAPTIN. J. Biol. Chem. 2002, 277, 9247–9254. [Google Scholar] [CrossRef] [PubMed]
- Goodman, O.B.; Krupnick, J.G.; Santini, F.; Gurevich, V.V.; Penn, R.B.; Gagnon, A.W.; Keen, J.H.; Benovic, J.L. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 1996, 383, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Raiborg, C.; Santini, F.; Keen, J.H.; Stenmark, H.; Benovic, J.L. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev. Cell 2003, 5, 709–722. [Google Scholar] [CrossRef]
- Marchese, A.; Benovic, J.L. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J. Biol. Chem. 2001, 276, 45509–45512. [Google Scholar] [CrossRef] [PubMed]
- Dores, M.R.; Trejo, J. Atypical regulation of G protein-coupled receptor intracellular trafficking by ubiquitination. Curr. Opin. Cell Biol. 2014, 27, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A. Endocytic trafficking of chemokine receptors. Curr. Opin. Cell Biol. 2014, 27, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Brzostowski, J.A.; Sawai, S.; Rozov, O.; Liao, X.-H.; Imoto, D.; Parent, C.A.; Kimmel, A.R. Phosphorylation of chemoattractant receptors regulates chemotaxis, actin reorganization and signal relay. J. Cell Sci. 2013, 126, 4614–4626. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Pittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waterhouse, C.C.M.; Beck, P.L.; Muruve, D.A.; Kubes, P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Zoudilova, M.; Kumar, P.; Ge, L.; Wang, P.; Bokoch, G.M.; DeFea, K.A. Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J. Biol. Chem. 2007, 282, 20634–20646. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.; Malik, M.; Ravyn, V.; Tomkowicz, B.; Ptasznik, A.; Collman, R.G. An arrestin-dependent multi-kinase signaling complex mediates MIP-1β/CCL4 signaling and chemotaxis of primary human macrophages. J. Leukoc. Biol. 2009, 86, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Greasley, P.J.; Clapham, J.C. Inverse agonism or neutral antagonism at G-protein coupled receptors: A medicinal chemistry challenge worth pursuing? Eur. J. Pharmacol. 2006, 553, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Meckel, T.; Brzostowski, J.A.; Yan, J.; Meier-Schellersheim, M.; Jin, T. Coupling mechanism of a GPCR and a heterotrimeric G protein during chemoattractant gradient sensing in dictyostelium. Sci. Signal. 2010, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Elzie, C.A.; Colby, J.; Sammons, M.A.; Janetopoulos, C. Dynamic localization of G proteins in Dictyostelium discoideum. J. Cell Sci. 2009, 122, 2597–2603. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Gaietta, G.; Bunemann, M.; Adams, S.R.; Oberdorff-Maass, S.; Behr, B.; Vilardaga, J.-P.; Tsien, R.Y.; Ellisman, M.H.; Lohse, M.J. A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat. Meth. 2005, 2, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Vilardaga, J.-P.; Bünemann, M.; Krasel, C.; Castro, M.; Lohse, M.J. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat. Biotechnol. 2003, 21, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Bünemann, M.; Frank, M.; Lohse, M.J. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. USA 2003, 100, 16077–16082. [Google Scholar] [CrossRef] [PubMed]
- Xu, X. Quantitative imaging of single live cells reveals spatiotemporal dynamics of multistep signaling events of chemoattractant gradient sensing in dictyostelium. Mol. Biol. Cell 2004, 16, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.J.; Nuber, S.; Hoffmann, C. Fluorescence/bioluminescence resonance energy transfer techniques to study G-protein-coupled receptor activation and signaling. Pharmacol. Rev. 2012, 64, 299–336. [Google Scholar] [CrossRef] [PubMed]
- Janetopoulos, C.; Jin, T.; Devreotes, P. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science 2001, 291, 2408–2411. [Google Scholar] [CrossRef] [PubMed]
- Siderovski, D.P.; Willard, F.S. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int. J. Biol. Sci. 2005, 1, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marcos, M.; Ghosh, P.; Farquhar, M.G. GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 3178–3183. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Murakami, H.; Asai, N.; Morone, N.; Watanabe, T.; Kawai, K.; Murakumo, Y.; Usukura, J.; Kaibuchi, K.; Takahashi, M. Akt/PKB regulates actin organization and cell motility via girdin/APE. Dev. Cell 2005, 9, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Garcia-Marcos, M.; Bornheimer, S.J.; Farquhar, M.G. Activation of Gαi3 triggers cell migration via regulation of GIV. J. Cell Biol. 2008, 182, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Enomoto, A.; Jijiwa, M.; Kato, T.; Hasegawa, T.; Ishida, M.; Sato, T.; Asai, N.; Murakumo, Y.; Takahashi, M. An actin-binding protein Girdin regulates the motility of breast cancer cells. Cancer Res. 2008, 68, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Tall, G.G. Ric-8 regulation of heterotrimeric G proteins. J. Recept. Signal Transduct. Res. 2013, 33, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Tall, G.G.; Krumins, A.M.; Gilman, A.G. Mammalian Ric-8A (synembryn) is a heterotrimeric Gα protein guanine nucleotide exchange factor. J. Biol. Chem. 2003, 278, 8356–8362. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, J.; Toro-Tapia, G.; Arriagada, C.; Riquelme, L.; Beyer, A.; Henriquez, J.P.; Caprile, T.; Mayor, R.; Marcellini, S.; Hinrichs, M.V.; et al. Ric-8A, a guanine nucleotide exchange factor for heterotrimeric G proteins, is critical for cranial neural crest cell migration. Dev. Biol. 2013, 378, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, D.; Xing, B.; Zhang, J.J.; Shu, H.-B.; Guo, L.; Huang, X.-Y. Resistance to inhibitors of cholinesterase-8A (Ric-8A) is critical for growth factor receptor-induced actin cytoskeletal reorganization. J. Biol. Chem. 2011, 286, 31055–31061. [Google Scholar] [CrossRef] [PubMed]
- Kataria, R.; Xu, X.; Fusetti, F.; Keizer-Gunnink, I.; Jin, T.; van Haastert, P.J.M.; Kortholt, A. Dictyostelium Ric8 is a nonreceptor guanine exchange factor for heterotrimeric G proteins and is important for development and chemotaxis. Proc. Natl. Acad. Sci. USA 2013, 110, 6424–6429. [Google Scholar] [CrossRef] [PubMed]
- Kataria, R.; van Haastert, P.J.M.; Kortholt, A. Reply to Tall et al.: Dictyostelium Ric8 does not have a chaperoning function during development and chemotaxis. Proc. Natl. Acad. Sci. USA 2013, 110, E3149. [Google Scholar] [CrossRef] [PubMed]
- Vellano, C.P.; Shu, F.-J.; Ramineni, S.; Yates, C.K.; Tall, G.G.; Hepler, J.R. Activation of the regulator of G protein signaling 14-Gαi1-GDP signaling complex is regulated by resistance to inhibitors of cholinesterase-8A. Biochemistry 2011, 50, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Druey, K.M.; Blumer, K.J.; Kang, V.H.; Kehrl, J.H. Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family. Nature 1996, 379, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, S.; Hepler, J.R. Cellular regulation of RGS proteins: Modulators and integrators of G protein signaling. Pharmacol. Rev. 2002, 54, 527–559. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.W.; Fields, T.A.; Casey, P.J.; Peralta, E.G. RGS10 is a selective activator of G alpha i GTPase activity. Nature 1996, 383, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, K.B.; Hepler, J.R. Cell signalling diversity of the Gqα family of heterotrimeric G proteins. Cell Signal. 2006, 18, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, D.E.; Kurachi, Y.; Galper, J.; Neer, E.J.; Clapham, D.E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 1987, 325, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Hepler, J.R.; Berman, D.M.; Gilman, A.G.; Kozasa, T. RGS4 and GAIP are GTPase-activating proteins for Gqα and block activation of phospholipase Cβ by γ-thio-GTP-Gq alpha. Proc. Natl. Acad. Sci. USA 1997, 94, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Firtel, R.A. A regulator of G protein signaling-containing kinase is important for chemotaxis and multicellular development in dictyostelium. Mol. Biol. Cell 2003, 14, 1727–1743. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.M.; Gilman, A.G. Mammalian RGS proteins: Barbarians at the gate. J. Biol. Chem. 1998, 273, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Albig, A.R.; Schiemann, W.P. Identification and characterization of regulator of G protein signaling 4 (RGS4) as a novel inhibitor of tubulogenesis: RGS4 inhibits mitogen-activated protein kinases and vascular endothelial growth factor signaling. Mol. Biol. Cell 2005, 16, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Huang, N.; Kim, D.; Kehrl, J.H. RGS1 and RGS13 mRNA silencing in a human B lymphoma line enhances responsiveness to chemoattractants and impairs desensitization. J. Leukoc. Biol. 2006, 79, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Sánche-Blázquez, P.; Rodríguez-Díaz, M.; López-Fando, A.; Rodríguez-Muñoz, M.; Garzo, J. The GBeta5 subunit that associates with the R7 subfamily of RGS proteins regulates mu-opioid effects. Neuropharmacology 2003, 45, 82–95. [Google Scholar]
- Willard, F.S.; Kimple, R.J.; Siderovski, D.P. Return of the GDI: The GoLoco motif in cell division. Annu. Rev. Biochem. 2004, 73, 925–951. [Google Scholar] [CrossRef] [PubMed]
- Kimple, R.J.; Willard, F.S.; Siderovski, D.P. The GoLoco motif: Heralding a new tango between G protein signaling and cell division. Mol. Interv. 2002, 2, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Glick, J.L.; Polakis, P.; Casey, P.J. Functional interaction between Galpha(z) and Rap1GAP suggests a novel form of cellular cross-talk. J. Biol. Chem. 1999, 274, 36663–36669. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Casey, P.J. Activation of Gz attenuates Rap1-mediated differentiation of PC12 cells. J. Biol. Chem. 2002, 277, 43417–43424. [Google Scholar] [CrossRef] [PubMed]
- De Vries, L.; Fischer, T.; Tronchère, H.; Brothers, G.M.; Strockbine, B.; Siderovski, D.P.; Farquhar, M.G. Activator of G protein signaling 3 is a guanine dissociation inhibitor for Galpha i subunits. Proc. Natl. Acad. Sci. USA 2000, 97, 14364–14369. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, S.; Nomura, M.; Hayase, J.; Iwakiri, Y.; Nishikimi, A.; Takayanagi, R.; Fukui, Y.; Sumimoto, H. The cell polarity protein minsc regulates neutrophil chemotaxis via a noncanonical G protein signaling pathway. Dev. Cell 2013, 26, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Willardson, B.M.; Howlett, A.C. Function of phosducin-like proteins in G protein signaling and chaperone-assisted protein folding. Cell Signal. 2007, 19, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.H.; Muller, S.; Puzicha, M.; Pippig, S.; Obermaier, B.; Helmreich, E.J.M.; Lohse, M.J. Phosducin is a protein kinase A-regulated G-protein regulator. Nature 1992, 358, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Dupré, D.J.; Robitaille, M.; Rebois, R.V.; Hébert, T.E. The Role of Gβγ Subunits in the organization, assembly, and function of GPCR signaling complexes. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, M.; Knol, J.C.; Kortholt, A.; Roelofs, J.; Postma, M.; Visser, A.J.; van Haastert, P.J. Phosducin-like proteins in Dictyostelium discoideum: Implications for the phosducin family of proteins. EMBO J. 2003, 22, 5047–5057. [Google Scholar] [CrossRef] [PubMed]
- Knol, J.C.; Engel, R.; Blaauw, M.; Visser, A.J.W.G.; van Haastert, P.J.M. The phosducin-like protein PhLP1 is essential for Gβγ dimer formation in dictyostelium discoideum. Mol. Cell. Biol. 2005, 25, 8393–8400. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, F.; Shin, M.E.; Wang, F.; Shen, L.; Hamm, H.E. RACK1 regulates directional cell migration by acting on Gβγ at the interface with its effectors PLCβ and PI3Kγ. Mol. Biol. Cell 2008, 19, 3909–3922. [Google Scholar] [CrossRef] [PubMed]
- Runne, C.; Chen, S. WD40-repeat proteins control the flow of Gβγ signaling for directional cell migration. Cell Adh. Migr. 2013, 7, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Surve, C.R.; Lehmann, D.; Smrcka, A.V. Chemical Biology Approach Demonstrates GTP-binding protein Gβγ subunits are sufficient to mediate directional neutrophil chemotaxis. J. Biol. Chem. 2014, 289, 17791–17801. [Google Scholar] [CrossRef] [PubMed]
- Runne, C.; Chen, S. PLEKHG2 promotes heterotrimeric G protein βγ-stimulated lymphocyte migration via Rac and Cdc42 activation and actin polymerization. Mol. Cell. Biol. 2013, 33, 4294–4307. [Google Scholar] [CrossRef] [PubMed]
- Swaney, K.F.; Huang, C.-H.; Devreotes, P.N. Eukaryotic chemotaxis: A network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 2010, 39, 265–289. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Amzel, M.; Devreotes, P.N.; Wu, L. Selection of Gβ subunits with point mutations that fail to activate specific signaling pathways in vivo: Dissecting cellular responses mediated by a heterotrimeric G protein in Dictyostelium discoideum. Mol. Biol. Cell 1998, 9, 2949–2961. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.T.; Chun, C.; Takeda, K.; Firtel, R.A. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J. Cell Biol. 2004, 167, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Stephens, L.; Milne, L.; Hawkins, P. Moving towards a better understanding of chemotaxis. Curr. Biol. 2008, 18, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Insall, R.; Kuspa, A.; Lilly, P.J.; Shaulsky, G.; Levin, L.R.; Loomis, W.F.; Devreotes, P. CRAC, a cytosolic protein containing a pleckstrin homology domain, is required for receptor and G protein-mediated activation of adenylyl cyclase in Dictyostelium. J. Cell Biol. 1994, 126, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Potikyan, G.; Firtel, R.A. Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol. Cell 2001, 7, 937–947. [Google Scholar] [CrossRef]
- Miki, H.; Suetsugu, S.; Takenawa, T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998, 17, 6932–6941. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Funamoto, S.; Milan, K.; Meili, R.; Firtel, R.A. Role of phosphatidylinositol 3′ kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in Dictyostelium. J. Cell Biol. 2001, 153, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Mihaylov, V.; Xu, X.; Brzostowski, J.A.; Li, H.; Liu, L.; Veenstra, T.D.; Parent, C.A.; Jin, T. A Gβγ Effector, ElmoE, Transduces GPCR signaling to the actin network during chemotaxis. Dev. Cell 2012, 22, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Mo, Z.; Bokoch, G.; Guo, C.; Li, Z.; Wu, D. P-Rex1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr. Biol. 2005, 15, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hannigan, M.; Mo, Z.; Liu, B.; Lu, W.; Wu, Y.; Smrcka, A.V.; Wu, G.; Li, L.; Liu, M.; Huang, C.K.; Wu, D. Directional sensing requires Gβγ-mediated PAK1 and PIXα-dependent activation of Cdc42. Cell 2003, 114, 215–227. [Google Scholar] [CrossRef]
- Brock, C. Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ. J. Cell Biol. 2002, 160, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, Y.; Nishikimi, A.; Tanaka, Y.; Takii, R.; Noda, M.; Inayoshi, A.; Watanabe, K.; Sanematsu, F.; Sasazuki, T.; Sasaki, T.; et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J. Cell Biol. 2006, 174, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, F. The signaling mechanisms underlying cell polarity and chemotaxis. Cold Spring Harb. Perspect. Biol. 2009, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, Y.; Xu, W.; Harden, T.K.; Sondek, J.; Sun, L.; Li, L.; Wu, D. A PLCβ/PI3Kγ-GSK3 Signaling pathway regulates cofilin phosphatase slingshot2 and neutrophil polarization and chemotaxis. Dev. Cell 2011, 21, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pipathsouk, A.; Keizer-Gunnink, A.; Fusetti, F.; Alkema, W.; Liu, S.; Altschuler, S.; Wu, L.; Kortholt, A.; Weiner, O.D. Homer3 regulates the establishment of neutrophil polarity. Mol. Biol. Cell 2015, 26, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, L.; Fu, H.; Yan, J.; Wang, Y.; Guo, H.; Hao, X.; Xu, X.; Jin, T.; Zhang, N. Association between Gαi2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis. Nat. Commun. 2013, 4, 1706. [Google Scholar] [CrossRef] [PubMed]
- Kozasa, T.; Jiang, X.; Hart, M.J.; Sternweis, P.M.; Singer, W.D.; Gilman, A.G.; Bollag, G.; Sternweis, P.C. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 1998, 280, 2109–2111. [Google Scholar] [CrossRef] [PubMed]
- Essler, M.; Amano, M.; Kruse, H.J.; Kaibuchi, K.; Weber, P.C.; Aepfelbacher, M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J. Biol. Chem. 1998, 273, 21867–21874. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Wang, J.; Wang, C.; Sommer, E.; Kozasa, T.; Srinivasula, S.; Alessi, D.; Offermanns, S.; Simon, M.I.; Wu, D. PRR5L degradation promotes mTORC2-mediated PKC-δ phosphorylation and cell migration downstream of Gα12. Nat. Cell Biol. 2012, 14, 686–696. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamp, M.E.; Liu, Y.; Kortholt, A. Function and Regulation of Heterotrimeric G Proteins during Chemotaxis. Int. J. Mol. Sci. 2016, 17, 90. https://doi.org/10.3390/ijms17010090
Kamp ME, Liu Y, Kortholt A. Function and Regulation of Heterotrimeric G Proteins during Chemotaxis. International Journal of Molecular Sciences. 2016; 17(1):90. https://doi.org/10.3390/ijms17010090
Chicago/Turabian StyleKamp, Marjon E., Youtao Liu, and Arjan Kortholt. 2016. "Function and Regulation of Heterotrimeric G Proteins during Chemotaxis" International Journal of Molecular Sciences 17, no. 1: 90. https://doi.org/10.3390/ijms17010090
APA StyleKamp, M. E., Liu, Y., & Kortholt, A. (2016). Function and Regulation of Heterotrimeric G Proteins during Chemotaxis. International Journal of Molecular Sciences, 17(1), 90. https://doi.org/10.3390/ijms17010090