Homeodomain-Interacting Protein Kinase-2: A Critical Regulator of the DNA Damage Response and the Epigenome
Abstract
:1. Introduction
2. Signaling Pathways for the DNA Damage Response (DDR)
3. Homeodomain-Interacting Protein Kinase 2 (HIPK2)
4. Regulation of HIPK2 Activities
5. Roles of HIPK2 in DDR
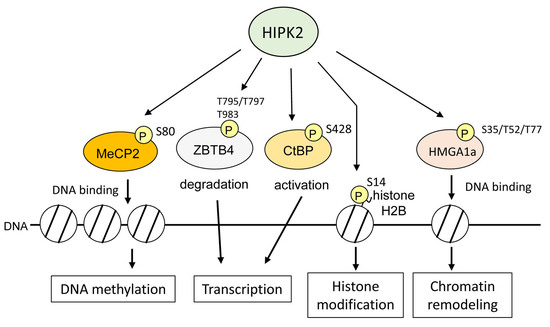
6. HIPK2 as an Epigenetic Regulator
7. A Role for HIPK2 in Notch1-Associated Tumorigenesis
8. Pathophysiological Functions of HIPK2
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.; Kastan, M.B. The DNA damage response: Implications for tumor responses to radiation and chemotherapy. Annu. Rev. Med. 2015, 66, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Elledge, S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.J. Targeting the DNA damage response in cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, N.M.; Clarkson, M.J.; Stuchbery, R.; Hovens, C.M. Molecular pathways: Targeting DNA repair pathway defects enriched in metastasis. Clin. Cancer Res. 2016, 22, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H. Genome maintenance mechanisms for preventing cancer. Nature 2001, 411, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Polo, S.E.; Almouzni, G. Chromatin dynamics after DNA damage: The legacy of the access-repair-restore model. DNA Repair 2015, 36, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Lu, X. Regulators in the DNA damage response. Arch. Biochem. Biophys. 2016, 594, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Matt, S.; Hofmann, T.G. The DNA damage-induced cell death response: A roadmap to kill cancer cells. Cell. Mol. Life Sci. 2016, 73, 2829–2850. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, S.; Rossi, A.; di Daniele, N.; Melino, G.; Annicchiarico-Petruzzelli, M.; Raschellà, G. DNA repair and aging: The impact of the p53 family. Aging 2015, 7, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Sulli, G.; Di Micco, R.; d’Adda di Fagagna, F. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat. Rev. Cancer 2012, 12, 709–720. [Google Scholar] [CrossRef] [PubMed]
- D’Orazi, G.; Cecchinelli, B.; Bruno, T.; Manni, I.; Higashimoto, Y.; Saito, S.; Gostissa, M.; Coen, S.; Marchetti, A.; del Sal, G.; et al. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat. Cell Biol. 2002, 4, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.G.; Möller, A.; Sirma, H.; Zentgraf, H.; Taya, Y.; Dröge, W.; Will, H.; Schmitz, M.L. Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nat. Cell Biol. 2002, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Papamichos-Chronakis, M.; Peterson, C.L. Chromatin and the genome integrity network. Nat. Rev. Genet. 2013, 14, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.H.; Stucki, M. Nucleolar responses to DNA double-strand breaks. Nucleic Acids Res. 2016, 44, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Tilgner, K.; Neganova, I.; Moreno-Gimeno, I.; Al-Aama, J.Y.; Burks, D.; Yung, S.; Singhapol, C.; Saretzki, G.; Evans, J.; Gorbunova, V.; et al. A human iPSC model of ligase IV deficiency reveals an important role for NHEJ-mediated-DSB repair in the survival and genomic stability of induced pluripotent stem cells and emerging haematopoietic progenitors. Cell Death Differ. 2013, 20, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Branzei, D.; Foiani, M. The DNA damage response during DNA replication. Curr. Opin. Cell Biol. 2005, 17, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, H.; García-Muse, T.; Aguilera, A. Replication stress and cancer. Nat. Rev. Cancer 2015, 15, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Moynahan, M.E.; Jasin, M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat. Rev. Mol. Cell Biol. 2010, 11, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Ceccaldi, R.; Liu, J.C.; Amunugama, R.; Hajdu, I.; Primack, B.; Petalcorin, M.I.; O’Connor, K.W.; Konstantinopoulos, P.A.; Elledge, S.J.; Boulton, S.J.; et al. Homologous-recombination-deficient tumours are dependent on polθ-mediated repair. Nature 2015, 518, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Gomez, P.A.; Gong, F.; Nair, N.; Miller, K.M.; Lazzerini-Denchi, E.; Sfeir, A. Mammalian polymerase θ promotes alternative NHEJ and suppresses recombination. Nature 2015, 518, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.; Wu, X.; Zhu, T.; Cheung, J.C.; Chen, H.; Lorincz, A.; Pandita, R.K.; Sharma, G.G.; Ha, H.C.; Gasson, J.; et al. A role for the HOXB7 homeodomain protein in DNA repair. Cancer Res. 2007, 67, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.K.; Jette, N.; Lees-Miller, S.P. Non-homologous end joining: Emerging themes and unanswered questions. DNA Repair 2014, 17, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.P.; Maser, R.S.; Olivares, H.; Davis, E.M.; Le Beau, M.; Yates, J.R.; Hays, L.; Morgan, W.F.; Petrini, J.H. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: Linkage of double-strand break repair to the cellular DNA damage response. Cell 1998, 93, 477–486. [Google Scholar] [CrossRef]
- Shiloh, Y. ATM and related protein kinases: Safeguarding genome integrity. Nat. Rev. Cancer 2003, 3, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, S.V.; Graham, M.E.; Jakob, B.; Tobias, F.; Kijas, A.W.; Tanuji, M.; Chen, P.; Robinson, P.J.; Taucher-Scholz, G.; Suzuki, K.; et al. Autophosphorylation and ATM activation: Additional sites add to the complexity. J. Biol. Chem. 2011, 286, 9107–9119. [Google Scholar] [CrossRef] [PubMed]
- Delacroix, S.; Wagner, J.M.; Kobayashi, M.; Yamamoto, K.; Karnitz, L.M. The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev. 2007, 21, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, C.Y.; Lee, S.J.; Conti, M.A.; Kim, Y. Homeodomain-interacting protein kinases, a novel family of co-repressors for homeodomain transcription factors. J. Biol. Chem. 1998, 273, 25875–25879. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, C.; Siepi, F.; Prodosmo, A.; Soddu, S. HIPKs: Jack of all trades in basic nuclear activities. Biochim. Biophys. Acta 2008, 1783, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Saul, V.V.; de la Vega, L.; Milanovic, M.; Krüger, M.; Braun, T.; Fritz-Wolf, K.; Becker, K.; Schmitz, M.L. HIPK2 kinase activity depends on cis-autophosphorylation of its activation loop. J. Mol. Cell Biol. 2013, 5, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, A.K.; Wei, G.; Doxakis, E.; Wong, C.; Tang, A.A.; Zang, K.; Luo, E.J.; Neve, R.L.; Reichardt, L.F.; Huang, E.J. Interaction of Brn3a and HIPK2 mediates transcriptional repression of sensory neuron survival. J. Cell Biol. 2004, 167, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Lu, Y.; Debbas, M.; Lin, A.W.; Sarosi, I.; Itie, A.; Wakeham, A.; Tuan, J.; Saris, C.; Elliott, G.; et al. Characterization of cells and gene-targeted mice deficient for the p53-binding kinase homeodomain-interacting protein kinase 1 (HIPK1). Proc. Natl. Acad. Sci. USA 2003, 100, 5431–5436. [Google Scholar] [CrossRef] [PubMed]
- Isono, K.; Nemoto, K.; Li, Y.; Takada, Y.; Suzuki, R.; Katsuki, M.; Nakagawara, A.; Koseki, H. Overlapping roles for homeodomain-interacting protein kinases HIPK1 and HIPK2 in the mediation of cell growth in response to morphogenetic and genotoxic signals. Mol. Cell. Biol. 2006, 26, 2758–2771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yoshimatsu, Y.; Hildebrand, J.; Frisch, S.M.; Goodman, R.H. Homeodomain interacting protein kinase 2 promotes apoptosis by downregulating the transcriptional corepressor CtBP. Cell 2003, 115, 177–186. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Puca, R.; Givol, D.; D’Orazi, G. HIPK2—A therapeutical target to be (re)activated for tumor suppression: Role in p53 activation and HIF-1α inhibition. Cell Cycle 2010, 9, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pho, V.; Bonasera, S.J.; Holtzman, J.; Tang, A.T.; Hellmuth, J.; Tang, S.; Janak, P.H.; Tecott, L.H.; Huang, E.J. Essential function of HIPK2 in TGFβ-dependent survival of midbrain dopamine neurons. Nat. Neurosci. 2007, 10, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ku, S.; Ma, G.K.; Saito, S.; Tang, A.A.; Zhang, J.; Mao, J.H.; Appella, E.; Balmain, A.; Huang, E.J. HIPK2 represses β-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 13040–13045. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Andrews, B.C.; Faust, M.; Walldorf, U.; Verheyen, E.M. HIPK is an essential protein that promotes notch signal transduction in the drosophila eye by inhibition of the global co-repressor groucho. Dev. Biol. 2009, 325, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.G.; Stollberg, N.; Schmitz, M.L.; Will, H. HIPK2 regulates transforming growth factor-β-induced c-Jun NH2-terminal kinase activation and apoptosis in human hepatoma cells. Cancer Res. 2003, 63, 8271–8277. [Google Scholar] [PubMed]
- Calzado, M.A.; Renner, F.; Roscic, A.; Schmitz, M.L. HIPK2: A versatile switchboard regulating the transcription machinery and cell death. Cell Cycle 2007, 6, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.G.; Glas, C.; Bitomsky, N. HIPK2: A tumour suppressor that controls DNA damage-induced cell fate and cytokinesis. Bioessays 2013, 35, 55–64. [Google Scholar] [CrossRef] [PubMed]
- De la Vega, L.; Hornung, J.; Kremmer, E.; Milanovic, M.; Schmitz, M.L. Homeodomain-interacting protein kinase 2-dependent repression of myogenic differentiation is relieved by its caspase-mediated cleavage. Nucleic Acids Res. 2013, 41, 5731–5745. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, Y.; Nguyen, L.A.; Isono, K.; Takakura, N.; Tagata, Y.; Schmitz, M.L.; Koseki, H.; Kitabayashi, I. Roles of HIPK1 and HIPK2 in AML1- and p300-dependent transcription, hematopoiesis and blood vessel formation. EMBO J. 2006, 25, 3955–3965. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Doan, C.N.; Arnold, T.D.; Lee, S.; Tang, A.A.; Reichardt, L.F.; Huang, E.J. Transcriptional corepressors HIPK1 and HIPK2 control angiogenesis via TGF-β–TAK1-dependent mechanism. PLoS Biol. 2013, 11, e1001527. [Google Scholar] [CrossRef] [PubMed]
- Sjölund, J.; Pelorosso, F.G.; Quigley, D.A.; DelRosario, R.; Balmain, A. Identification of HIPK2 as an essential regulator of white fat development. Proc. Natl. Acad. Sci. USA 2014, 111, 7373–7378. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, C.; Prodosmo, A.; Siepi, F.; Rinaldo, C.; Galli, F.; Gentileschi, M.; Bartolazzi, A.; Costanzo, A.; Sacchi, A.; Guerrini, L.; et al. HIPK2 phosphorylates ΔNp63α and promotes its degradation in response to DNA damage. Oncogene 2011, 30, 4802–4813. [Google Scholar] [CrossRef] [PubMed]
- Pierantoni, G.M.; Esposito, F.; Tornincasa, M.; Rinaldo, C.; Viglietto, G.; Soddu, S.; Fusco, A. Homeodomain-interacting protein kinase-2 stabilizes p27kip1 by its phosphorylation at serine 10 and contributes to cell motility. J. Biol. Chem. 2011, 286, 29005–29013. [Google Scholar] [CrossRef] [PubMed]
- Gresko, E.; Roscic, A.; Ritterhoff, S.; Vichalkovski, A.; del Sal, G.; Schmitz, M.L. Autoregulatory control of the p53 response by caspase-mediated processing of HIPK2. EMBO J. 2006, 25, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, C.; Moncada, A.; Gradi, A.; Ciuffini, L.; D’Eliseo, D.; Siepi, F.; Prodosmo, A.; Giorgi, A.; Pierantoni, G.M.; Trapasso, F.; et al. HIPK2 controls cytokinesis and prevents tetraploidization by phosphorylating histone H2B at the midbody. Mol. Cell 2012, 47, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W.; Seo, Y.M.; Kim, E.A.; Sung, K.S.; Ahn, J.W.; Park, S.J.; Lee, S.R.; Choi, C.Y. Ubiquitination and degradation of homeodomain-interacting protein kinase 2 by WD40 repeat/SOCS box protein Wsb-1. J. Biol. Chem. 2008, 283, 4682–4689. [Google Scholar] [CrossRef] [PubMed]
- Boucher, M.J.; Simoneau, M.; Edlund, H. The homeodomain-interacting protein kinase 2 regulates insulin promoter factor-1/pancreatic duodenal homeobox-1 transcriptional activity. Endocrinology 2009, 150, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Roscic, A.; Möller, A.; Calzado, M.A.; Renner, F.; Wimmer, V.C.; Gresko, E.; Lüdi, K.S.; Schmitz, M.L. Phosphorylation-dependent control of Pc2 SUMO E3 ligase activity by its substrate protein HIPK2. Mol. Cell 2006, 24, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Conrad, E.; Polonio-Vallon, T.; Meister, M.; Matt, S.; Bitomsky, N.; Herbel, C.; Liebl, M.; Greiner, V.; Kriznik, B.; Schumacher, S.; et al. HIPK2 restricts Sirt1 activity upon severe DNA damage by a phosphorylation-controlled mechanism. Cell Death Differ. 2016, 23, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Sombroek, D.; Dauth, I.; Moehlenbrink, J.; Scheuermann, K.; Crone, J.; Hofmann, T.G. Control of HIPK2 stability by ubiquitin ligase Siah-1 and checkpoint kinases ATM and ATR. Nat. Cell Biol. 2008, 10, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Blandino, G.; Sacchi, A.; Soddu, S.; D’Orazi, G. HIPK2 neutralizes MDM2 inhibition rescuing p53 transcriptional activity and apoptotic function. Oncogene 2004, 23, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, S.; Coulibaly, A.; Ohnheiser, J.; Jakobs, A.; Klempnauer, K.H. Interaction and cooperation of the CCAAT-box enhancer-binding protein β (C/EBPβ) with the homeodomain-interacting protein kinase 2 (HIPK2). J. Biol. Chem. 2013, 288, 22257–22269. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, F.; Catena, V.; Rinaldo, C.; Bruno, T.; Iezzi, S.; Sorino, C.; Desantis, A.; Camerini, S.; Crescenzi, M.; Floridi, A.; et al. HIPK2 sustains apoptotic response by phosphorylating Che-1/AATF and promoting its degradation. Cell Death Dis. 2014, 5, e1414. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, G.; Conca, B.; Bergo, A.; Rusconi, L.; Zhou, Z.; Greenberg, M.E.; Landsberger, N.; Soddu, S.; Kilstrup-Nielsen, C. Methyl-CpG-binding protein 2 is phosphorylated by homeodomain-interacting protein kinase 2 and contributes to apoptosis. EMBO Rep. 2009, 10, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Yamada, D.; Pérez-Torrado, R.; Filion, G.; Caly, M.; Jammart, B.; Devignot, V.; Sasai, N.; Ravassard, P.; Mallet, J.; Sastre-Garau, X.; et al. The human protein kinase HIPK2 phosphorylates and downregulates the methyl-binding transcription factor ZBTB4. Oncogene 2009, 28, 2535–2544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y. Homeodomain-interacting protein kinase-2 (HIPK2) phosphorylates HMGA1a at Ser-35, Thr-52, and Thr-77 and modulates its DNA binding affinity. J. Proteome Res. 2007, 6, 4711–4719. [Google Scholar] [CrossRef] [PubMed]
- Akaike, Y.; Kuwano, Y.; Nishida, K.; Kurokawa, K.; Kajita, K.; Kano, S.; Masuda, K.; Rokutan, K. Homeodomain-interacting protein kinase 2 regulates DNA damage response through interacting with heterochromatin protein 1γ. Oncogene 2015, 34, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Bitomsky, N.; Conrad, E.; Moritz, C.; Polonio-Vallon, T.; Sombroek, D.; Schultheiss, K.; Glas, C.; Greiner, V.; Herbel, C.; Mantovani, F.; et al. Autophosphorylation and Pin1 binding coordinate DNA damage-induced HIPK2 activation and cell death. Proc. Natl. Acad. Sci. USA 2013, 110, E4203–E4212. [Google Scholar] [CrossRef] [PubMed]
- Shima, Y.; Shima, T.; Chiba, T.; Irimura, T.; Pandolfi, P.P.; Kitabayashi, I. PML activates transcription by protecting HIPK2 and p300 from SCFFbx3-mediated degradation. Mol. Cell. Biol. 2008, 28, 7126–7138. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.G.; Jaffray, E.; Stollberg, N.; Hay, R.T.; Will, H. Regulation of homeodomain-interacting protein kinase 2 (HIPK2) effector function through dynamic small ubiquitin-related modifier-1 (SUMO-1) modification. J. Biol. Chem. 2005, 280, 29224–29232. [Google Scholar] [CrossRef] [PubMed]
- De la Vega, L.; Grishina, I.; Moreno, R.; Krüger, M.; Braun, T.; Schmitz, M.L. A redox-regulated SUMO/acetylation switch of HIPK2 controls the survival threshold to oxidative stress. Mol. Cell 2012, 46, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Cano, C.E.; Gommeaux, J.; Pietri, S.; Culcasi, M.; Garcia, S.; Seux, M.; Barelier, S.; Vasseur, S.; Spoto, R.P.; Pébusque, M.J.; et al. Tumor protein 53-induced nuclear protein 1 is a major mediator of p53 antioxidant function. Cancer Res. 2009, 69, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hailemariam, K.; Iwasaki, K.; Huang, B.W.; Sakamoto, K.; Tsuji, Y. Transcriptional regulation of ferritin and antioxidant genes by HIPK2 under genotoxic stress. J. Cell Sci. 2010, 123, 3863–3871. [Google Scholar] [CrossRef] [PubMed]
- Nikitaki, Z.; Mavragani, I.V.; Laskaratou, D.A.; Gika, V.; Moskvin, V.P.; Theofilatos, K.; Vougas, K.; Stewart, R.D.; Georgakilas, A.G. Systemic mechanisms and effects of ionizing radiation: A new ”old” paradigm of how the bystanders and distant can become the players. Semin. Cancer Biol. 2016, 37, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Dauth, I.; Krüger, J.; Hofmann, T.G. Homeodomain-interacting protein kinase 2 is the ionizing radiation-activated p53 serine 46 kinase and is regulated by ATM. Cancer Res. 2007, 67, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Reuven, N.; Adler, J.; Porat, Z.; Polonio-Vallon, T.; Hofmann, T.G.; Shaul, Y. The tyrosine kinase c-Abl promotes homeodomain-interacting protein kinase 2 (HIPK2) accumulation and activation in response to DNA damage. J. Biol. Chem. 2015, 290, 16478–16488. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Soddu, S.; Sacchi, A.; D’Orazi, G. HIPK2 contributes to PCAF-mediated p53 acetylation and selective transactivation of p21Waf1 after nonapoptotic DNA damage. Oncogene 2005, 24, 5431–5442. [Google Scholar] [CrossRef] [PubMed]
- Möller, A.; Sirma, H.; Hofmann, T.G.; Rueffer, S.; Klimczak, E.; Dröge, W.; Will, H.; Schmitz, M.L. PML is required for homeodomain-interacting protein kinase 2 (HIPK2)-mediated p53 phosphorylation and cell cycle arrest but is dispensable for the formation of HIPK domains. Cancer Res. 2003, 63, 4310–4314. [Google Scholar] [PubMed]
- Gresko, E.; Ritterhoff, S.; Sevilla-Perez, J.; Roscic, A.; Fröbius, K.; Kotevic, I.; Vichalkovski, A.; Hess, D.; Hemmings, B.A.; Schmitz, M.L. PML tumor suppressor is regulated by HIPK2-mediated phosphorylation in response to DNA damage. Oncogene 2009, 28, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, H.; Dessain, S.K.; Ng Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Bestor, T.H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 2000, 9, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Filion, G.J.; Zhenilo, S.; Salozhin, S.; Yamada, D.; Prokhortchouk, E.; Defossez, P.A. A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol. Cell. Biol. 2006, 26, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Oulad-Abdelghani, M.; Ortiz, J.A.; Remboutsika, E.; Chambon, P.; Losson, R. Heterochromatin formation in mammalian cells: Interaction between histones and HP1 proteins. Mol. Cell 2001, 7, 729–739. [Google Scholar] [CrossRef]
- Canzio, D.; Larson, A.; Narlikar, G.J. Mechanisms of functional promiscuity by HP1 proteins. Trends Cell Biol. 2014, 24, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.; Jeyasekharan, A.D.; Bernal, J.A.; Venkitaraman, A.R. HP1β mobilization promotes chromatin changes that initiate the DNA damage response. Nature 2008, 453, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Luijsterburg, M.S.; Dinant, C.; Lans, H.; Stap, J.; Wiernasz, E.; Lagerwerf, S.; Warmerdam, D.O.; Lindh, M.; Brink, M.C.; Dobrucki, J.W.; et al. Heterochromatin protein 1 is recruited to various types of DNA damage. J. Cell Biol. 2009, 185, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed]
- Puca, R.; Nardinocchi, L.; Givol, D.; D’Orazi, G. Regulation of p53 activity by HIPK2: Molecular mechanisms and therapeutical implications in human cancer cells. Oncogene 2010, 29, 4378–4387. [Google Scholar] [CrossRef] [PubMed]
- Ann, E.J.; Kim, M.Y.; Yoon, J.H.; Ahn, J.S.; Jo, E.H.; Lee, H.J.; Lee, H.W.; Kang, H.G.; Choi, D.W.; Chun, K.H.; et al. Tumor suppressor HIPK2 regulates malignant growth via phosphorylation of Notch1. Cancer Res. 2016, 76, 4728–4740. [Google Scholar] [CrossRef] [PubMed]
- Pateras, I.S.; Havaki, S.; Nikitopoulou, X.; Vougas, K.; Townsend, P.A.; Panayiotidis, M.I.; Georgakilas, A.G.; Gorgoulis, V.G. The DNA damage response and immune signaling alliance: Is it good or bad? Nature decides when and where. Pharmacol. Ther. 2015, 154, 36–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moiseeva, O.; Mallette, F.A.; Mukhopadhyay, U.K.; Moores, A.; Ferbeyre, G. DNA damage signaling and p53-dependent senescence after prolonged β-interferon stimulation. Mol. Biol. Cell 2006, 17, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S. Nuclear initiated NF-κB signaling: NEMO and ATM take center stage. Cell Res. 2011, 21, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Sabatel, H.; di Valentin, E.; Gloire, G.; Dequiedt, F.; Piette, J.; Habraken, Y. Phosphorylation of p65(RelA) on Ser547 by atm represses NF-κB-dependent transcription of specific genes after genotoxic stress. PLoS ONE 2012, 7, e38246. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tong, J.; He, F.; Yu, X.; Fan, L.; Hu, J.; Tan, J.; Chen, Z. miR-141 regulates TGF-β1-induced epithelial-mesenchymal transition through repression of HIPK2 expression in renal tubular epithelial cells. Int. J. Mol. Med. 2015, 35, 311–318. [Google Scholar] [CrossRef] [PubMed]




| Target Proteins | Cellular Function | Reference |
|---|---|---|
| p53 | cell cycle regulation and apoptosis | [13,14,33] |
| ΔNp63α | cell cycle regulation and apoptosis | [47] |
| p27 | cell cycle regulation and cell motility | [48] |
| PML | cell proliferation | [49] |
| H2B | cytokinesis | [50] |
| WIP1 | DNA repair | [51] |
| PDX1 | pancreatic development and mature β-cell function | [52] |
| β-catenin | activation of Wnt/β-catenin signaling | [36] |
| Pc2 | SUMOylation | [53] |
| SIRT1 | acetylation | [54] |
| Siah1, Siah2 | ubiquitination | [55] |
| MDM2 | ubiquitination | [56] |
| C/EBPβ | activation of transcription factor | [57] |
| CtBP | transcription corepression | [35] |
| p300, AML1 | transcription activation; hematopoiesis | [44] |
| Che-1 | transcription activation; cell proliferation | [58] |
| DAXX | transcription repression | [40] |
| MeCP2 | DNA methylation and epigenetic regulation | [59] |
| ZBTB4 | transcription and epigenetic regulation | [60] |
| HMGA1a | transcriptional regulation and chromatin remodeling | [61] |
| HP1γ | epigenetic regulation | [62] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuwano, Y.; Nishida, K.; Akaike, Y.; Kurokawa, K.; Nishikawa, T.; Masuda, K.; Rokutan, K. Homeodomain-Interacting Protein Kinase-2: A Critical Regulator of the DNA Damage Response and the Epigenome. Int. J. Mol. Sci. 2016, 17, 1638. https://doi.org/10.3390/ijms17101638
Kuwano Y, Nishida K, Akaike Y, Kurokawa K, Nishikawa T, Masuda K, Rokutan K. Homeodomain-Interacting Protein Kinase-2: A Critical Regulator of the DNA Damage Response and the Epigenome. International Journal of Molecular Sciences. 2016; 17(10):1638. https://doi.org/10.3390/ijms17101638
Chicago/Turabian StyleKuwano, Yuki, Kensei Nishida, Yoko Akaike, Ken Kurokawa, Tatsuya Nishikawa, Kiyoshi Masuda, and Kazuhito Rokutan. 2016. "Homeodomain-Interacting Protein Kinase-2: A Critical Regulator of the DNA Damage Response and the Epigenome" International Journal of Molecular Sciences 17, no. 10: 1638. https://doi.org/10.3390/ijms17101638
APA StyleKuwano, Y., Nishida, K., Akaike, Y., Kurokawa, K., Nishikawa, T., Masuda, K., & Rokutan, K. (2016). Homeodomain-Interacting Protein Kinase-2: A Critical Regulator of the DNA Damage Response and the Epigenome. International Journal of Molecular Sciences, 17(10), 1638. https://doi.org/10.3390/ijms17101638