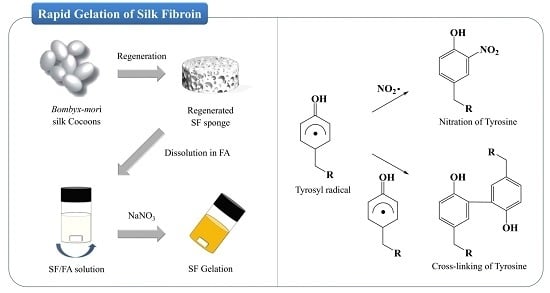
Gelation Behaviors and Mechanism of Silk Fibroin According to the Addition of Nitrate Salts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gelation Behavior of Silk Fibroin (SF)/Formic Acid Solution According to the Addition of a Variety of Salts
2.2. Effect of SF and NaNO3 Concentration on Gelation
2.3. Viscosity Change According to the NaNO3 Concentration
2.4. Compositional Change of SF upon Gelation
2.4.1. Amino Acid Analysis
2.4.2. UV-Vis Spectroscopy
2.5. Fluorescence Spectroscopy
2.6. Mechanism of SF Gelation and Nitration
3. Materials and Methods
3.1. Materials
3.2. Preparation of SF/Formic Acid Solution
3.3. Gelation Behavior of SF/Formic Acid Solution According to the Addition of a Variety of Salts
3.4. Characterization
3.4.1. Viscosity Change Depending on the NaNO3 Concentration
3.4.2. Amino Acid Analysis
3.4.3. UV-Vis Spectrophotometry
3.4.4. Fluorescence Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jin, H.-J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Seidel, A.; Liivak, O.; Calve, S.; Adaska, J.; Ji, G.; Yang, Z.; Grubb, D.; Zax, D.B.; Jelinski, L.W. Regenerated spider silk: Processing, properties, and structure. Macromolecules 2000, 33, 775–780. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Gunawidjaja, R.; Lin, Y.H.; Gupta, M.K.; Kaplan, D.L.; Naik, R.R.; Tsukruk, V.V. Mechanical properties of robust ultrathin silk fibroin films. Adv. Funct. Mater. 2007, 17, 2229–2237. [Google Scholar] [CrossRef]
- Hunt, S. Amino acid composition of silk from the pseudoscorpion Neobisium maritimum (Leach): A possible link between the silk fibroins and the keratins. Comp. Biochem. Physiol. 1970, 34, 773–776. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Mo, X.-M.; Zhang, K.-H.; Fan, L.-P.; Yin, A.-L.; He, C.-L.; Wang, H.-S. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Motta, A.; Freddi, G.; Cannas, M. In vitro evaluation of the inflammatory potential of the silk fibroin. J. Biomed. Mater. Res. 1999, 46, 382–389. [Google Scholar] [CrossRef]
- Kim, U.-J.; Park, J.; Kim, H.J.; Wada, M.; Kaplan, D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005, 26, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Foo, C.W.P.; Rossetti, F.; Textor, M.; Vunjak-Novakovic, G.; Kaplan, D.; Merkle, H.; Meinel, L. Silk fibroin as an organic polymer for controlled drug delivery. J. Control. Release 2006, 111, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M. The silk proteins, sericin and fibroin in silkworm, Bombyx mori Linn.—A review. Casp. J. Environ. Sci. 2007, 5, 63–76. [Google Scholar]
- Schroeder, W.; Kay, L.M.; Lewis, B.; Munger, N. The amino acid composition of Bombyx mori silk fibroin and of tussah silk fibroin. J. Am. Chem. Soc. 1955, 77, 3908–3913. [Google Scholar] [CrossRef]
- Fossey, S.A.; Némethy, G.; Gibson, K.D.; Scheraga, H.A. Conformational energy studies of β-sheets of model silk fibroin peptides. I. Sheets of poly (Ala-Gly) chains. Biopolymers 1991, 31, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, W.H. Chemically cross-linked silk fibroin hydrogel with enhanced elastic properties, biodegradability, and biocompatibility. Int. J. Nanomed. 2016, 11, 2967. [Google Scholar]
- Yamada, H.; Nakao, H.; Takasu, Y.; Tsubouchi, K. Preparation of undegraded native molecular fibroin solution from silkworm cocoons. Mater. Sci. Eng. C 2001, 14, 41–46. [Google Scholar] [CrossRef]
- Padamwar, M.; Pawar, A. Silk sericin and its applications: A review. J. Sci. Ind. Res. 2004, 63, 323–329. [Google Scholar]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, R.; Jin, H.-J.; Kaplan, D.L. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules 2004, 5, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, S.; Lele, A.; Chassenieux, C.; Nicolai, T.; Durand, D.; Co, A.; Leal, G.L.; Colby, R.H.; Giacomin, A.J. Gelation of Regenerated Fibroin Solution. In Aip Conference Proceedings; AIP: Monterey, CA, USA, 2008; p. 573. [Google Scholar]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D.L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hou, J.; Li, M.; Wang, J.; Kaplan, D.L.; Lu, S. Sodium dodecyl sulfate-induced rapid gelation of silk fibroin. Acta Biomater. 2012, 8, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Jeong, L.; Cho, D.; Kwon, O.H.; Park, W.H. Effect of methylcellulose on the formation and drug release behavior of silk fibroin hydrogel. Carbohydr. Polym. 2013, 98, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; He, J.; Nichol, J.W.; Wang, L.; Hutson, C.B.; Wang, B.; Du, Y.; Fan, H.; Khademhosseini, A. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011, 7, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Y.; Pöschl, U. Quantification of nitrotyrosine in nitrated proteins. Anal. Bioanal. Chem. 2010, 397, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L.; Choudhury, N.R.; Dutta, N.K.; Zannettino, A. Facile and rapid ruthenium mediated photo-crosslinking of Bombyx mori silk fibroin. J. Mater. Chem. B 2014, 2, 6259–6270. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Schmidt, K.; Mayer, B. Dityrosine formation outcompetes tyrosine nitration at low steady-state concentrations of peroxynitrite implications for tyrosine modification by nitric oxide/superoxide in vivo. J. Biol. Chem. 2000, 275, 6346–6352. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, T.; Giulivi, C. Current analytical methods for the detection of dityrosine, a biomarker of oxidative stress, in biological samples. Mass Spectrom. Rev. 2007, 26, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Van der Vliet, A.; Eiserich, J.P.; Shigenaga, M.K.; Cross, C.E. Reactive nitrogen species and tyrosine nitration in the respiratory tract: Epiphenomena or a pathobiologic mechanism of disease? Am. J. Respir. Crit. Care Med. 1999, 160, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sah, M.; Pramanik, K. Regenerated silk fibroin from B. mori silkcocoon for tissue engineering applications. Int. J. Environ. Sci. Dev. 2010, 1, 404. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.; Park, Y.H.; Hudson, S. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int. J. Biol. Macromol. 2001, 29, 91–97. [Google Scholar] [CrossRef]
- Kim, H.H.; Song, D.W.; Kim, M.J.; Ryu, S.J.; Um, I.C.; Ki, C.S.; Park, Y.H. Effect of silk fibroin molecular weight on physical property of silk hydrogel. Polymer 2016, 90, 26–33. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.; Li, M. Silk fibroin based porous materials. Materials 2009, 2, 2276–2295. [Google Scholar] [CrossRef]
- Crow, J.P.; Beckman, J.S. Quantitation of Protein Tyrosine, 3-Nitrotyrosine, and 3-Aminotyrosine Utilizing HPLC and Intrinsic Ultrviolet Absorbance. Methods 1995, 7, 116–120. [Google Scholar] [CrossRef]











© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, D.S.; Kim, M.H.; Yoon, Y.I.; Park, W.H. Gelation Behaviors and Mechanism of Silk Fibroin According to the Addition of Nitrate Salts. Int. J. Mol. Sci. 2016, 17, 1697. https://doi.org/10.3390/ijms17101697
Im DS, Kim MH, Yoon YI, Park WH. Gelation Behaviors and Mechanism of Silk Fibroin According to the Addition of Nitrate Salts. International Journal of Molecular Sciences. 2016; 17(10):1697. https://doi.org/10.3390/ijms17101697
Chicago/Turabian StyleIm, Dong Su, Min Hee Kim, Young Il Yoon, and Won Ho Park. 2016. "Gelation Behaviors and Mechanism of Silk Fibroin According to the Addition of Nitrate Salts" International Journal of Molecular Sciences 17, no. 10: 1697. https://doi.org/10.3390/ijms17101697
APA StyleIm, D. S., Kim, M. H., Yoon, Y. I., & Park, W. H. (2016). Gelation Behaviors and Mechanism of Silk Fibroin According to the Addition of Nitrate Salts. International Journal of Molecular Sciences, 17(10), 1697. https://doi.org/10.3390/ijms17101697