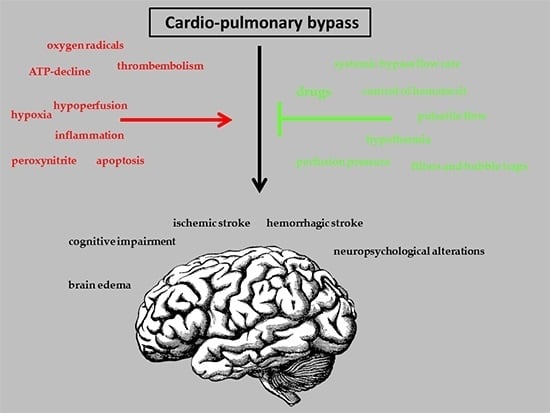
Neuroprotective Strategies during Cardiac Surgery with Cardiopulmonary Bypass
Abstract
:1. Introduction
2. Apparative Methods for Brain Protection
3. Pharmacological Methods for Brain Protection
3.1. Anti-Inflammatory Drugs
3.2. Miscellaneous Drugs
4. Summary
Conflicts of Interest
References
- Elsberg, C.A. An experimental investigation of the treatment of wounds of the heart by means of suture of the heart muscle. J. Exp. Med. 1899, 4, 479–520. [Google Scholar] [CrossRef] [PubMed]
- Gibbon, J.H., Jr. The application of a mechanical heart and lung apparatus to cardiac surgery. Minn. Med. 1954, 37, 171–185. [Google Scholar] [PubMed]
- Bigelow, W.G. Application of hypothermia to cardiac surgery. Minn. Med. 1954, 37, 181–185. [Google Scholar] [PubMed]
- Zamvar, V.; Williams, D.; Hall, J.; Payne, N.; Cann, C.; Young, K.; Karthikeyan, S.; Dunne, J. Assessment of neurocognitive impairment after off-pump and on-pump techniques for coronary artery bypass graft surgery: Prospective randomised controlled trial. BMJ 2002, 325, 1268. [Google Scholar] [CrossRef] [PubMed]
- Bayram, H.; Hidiroglu, M.; Cetin, L.; Kucuker, A.; Iriz, E.; Uguz, E.; Saglam, F.; Sener, E. Comparing S-100β protein levels and neurocognitive functions between patients undergoing on-pump and off-pump coronary artery bypass grafting. J. Surg. Res. 2013, 182, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Stroobant, N.; van Nooten, G.; Belleghem, Y.; Vingerhoets, G. Short-term and long-term neurocognitive outcome in on-pump versus off-pump CABG. Eur. J. Cardiothorac. Surg. 2002, 22, 559–564. [Google Scholar] [CrossRef]
- Newman, M.F.; Kirchner, J.L.; Phillips-Bute, B.; Gaver, V.; Grocott, H.; Jones, R.H.; Mark, D.B.; Reves, J.G.; Blumenthal, J.A. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N. Engl. J. Med. 2001, 344, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Bucerius, J.; Gummert, J.F.; Borger, M.A.; Walther, T.; Doll, N.; Onnasch, J.F.; Metz, S.; Falk, V.; Mohr, F.W. Stroke after cardiac surgery: A risk factor analysis of 16,184 consecutive adult patients. Ann. Thorac. Surg. 2003, 75, 472–478. [Google Scholar] [CrossRef]
- Prasongsukarn, K.; Borger, M.A. Reducing cerebral emboli during cardiopulmonary bypass. Semin. Cardiothorac. Vasc. Anesth. 2005, 9, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, D.; Jansen, E.W.; Hijman, R.; Nierich, A.P.; Diephuis, J.C.; Moons, K.G.; Lahpor, J.R.; Borst, C.; Keizer, A.M.; Nathoe, H.M.; et al. Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: A randomized trial. JAMA 2002, 287, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.O.; Rasmussen, L.S.; Steinbrüchel, D.A. Cognitive outcomes in elderly high-risk patients 1 year after off-pump versus on-pump coronary artery bypass grafting. A randomized trial. Eur. J. Cardiothorac. Surg. 2008, 34, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.A.; Andrew, M.J.; Ross, I.K.; Knight, J.L. The Octopus II stabilizing system: Biochemical and neuropsychological outcomes in coronary artery bypass surgery. Heart Surg. Forum 2001, 4 (Suppl. 1), S19–S23. [Google Scholar] [PubMed]
- Kennedy, E.D.; Choy, K.C.; Alston, R.P.; Chen, S.; Farhan-Alanie, M.M.; Anderson, J.; Ang, Y.L.; Moore, D.E.; Mackenzie, S.A.; Sykes, R.A. Cognitive outcome after on- and off-pump coronary artery bypass grafting surgery: A systematic review and meta-analysis. J. Cardiothorac. Vasc. Anesth. 2013, 27, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.C.; Bainbridge, D.; Martin, J.E.; Novick, R.J.; Evidence-Based Perioperative Clinical Outcomes Research Group. Does off-pump coronary artery bypass reduce mortality, morbidity, and resource utilization when compared with conventional coronary artery bypass? A meta-analysis of randomized trials. Anesthesiology 2005, 102, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.J.; Angelini, G.D. Side effects of cardiopulmonary bypass: What is the reality? J. Card. Surg. 2004, 19, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Stroobant, N.; van Nooten, G.; van Belleghem, Y.; Vingerhoets, G. The effect of CABG on neurocognitive functioning. Acta Cardiol. 2010, 65, 557–564. [Google Scholar] [PubMed]
- Bayliss, W.M. On the local reactions of the arterial wall to changes of internal pressure. J. Physiol. 1902, 28, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Kusch, B.; Vogt, S.; Sirat, A.S.; Helwig-Rohlig, A.; Kasseckert, S.; Moosdorf, R. Serum S-100β protein release in coronary artery bypass grafting: Laminar versus pulsatile flow. Thorac. Cardiovasc. Surg. 2001, 49, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Özatik, M.A.; Tarcan, O.; Kale, A.; Aşkin, G.A.; Balci, M.; Undar, A.; Küçükaksu, D.S.; Sener, E.; Taşdemir, O. Do S100β protein level increases due to inflammation during cardiopulmonary bypass occur without any neurological deficit? Perfusion 2002, 17, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Demir, T.; Demir, H.; Tansel, T.; Kalko, Y.; Tireli, E.; Dayioglu, E.; Barlas, S.; Onursal, E. Influence of methylprednisolone on levels of neuron-specific enolase in cardiac surgery: A corticosteroid derivative to decrease possible neuronal damage. J. Card. Surg. 2009, 24, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sharma, G.; Mishra, V. Hypoxia inducible factor-1: Its potential role in cerebral ischemia. Cell. Mol. Neurobiol. 2012, 32, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cox, S.R.; Morita, T.; Kourembanas, S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 1995, 77, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Ebert, B.L.; Firth, J.D.; Ratcliffe, P.J. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct cis-acting sequences. J. Biol. Chem. 1995, 270, 29083–29089. [Google Scholar] [PubMed]
- Manalo, D.J.; Rowan, A.; Lavoie, T.; Natarajan, L.; Kelly, B.D.; Ye, S.Q.; Garcia, J.G.; Semenza, G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005, 105, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factor 1: Oxygen homeostasis and disease pathophysiology. Trends. Mol. Med. 2001, 7, 345–350. [Google Scholar] [CrossRef]
- Althaus, J.; Bernaudin, M.; Petit, E.; Toutain, J.; Touzani, O.; Rami, A. Expression of the gene encoding the pro-apoptotic BNIP3 protein and stimulation of hypoxia-inducible factor-1alpha (HIF-1α) protein following focal cerebral ischemia in rats. Neurochem. Int. 2006, 48, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Dor, Y.; Herbert, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.J.; Gao, J.; Ma, X.B.; Yan, K.; Liu, X.X.; Kang, H.F.; Ji, Z.Z.; Guan, H.T.; Wang, X.J. Upregulation of hypoxia inducible factor-1α by cobalt chloride correlates with proliferation and apoptosis in PC-2 cells. J. Exp. Clin. Cancer. Res. 2012, 31, 28. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Abdul, K.S.; Jovanović, S.; Du, Q.; Sukhodub, A.; Jovanović, A. Mild hypoxia in vivo regulates cardioprotective SUR2A: A role for Akt and LDH. Biochim. Biophys. Acta 2015, 1852, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Abdul, K.S.; Jovanović, S.; Sukhodub, A.; Du, Q.; Jovanović, A. Upregulation of cardioprotective SUR2A by sub-hypoxic drop in oxygen. Biochim. Biophys. Acta 2014, 1843, 2424–2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simard, J.M.; Chen, M.; Tarasov, K.V.; Bhatta, S.; Ivanova, S.; Melnitchenko, L.; Tsymbalyuk, N.; West, G.A.; Gerzanich, V. Newly expressed SUR1-regulated NCCa-ATP channel mediates cerebral edema after ischemic stroke. Nat. Med. 2006, 12, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Gimeno-Bayon, J.; Espinosa-Parrilla, J.F.; Carrasco, J.L.; Batlle, M.; Pugliese, M.; Mahy, N.; Rodríguez, M.J. ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats. Exp. Neurol. 2012, 235, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Borutaite, V.; Toleikis, A.; Brown, G.C. In the eye of the storm: Mitochondrial damage during heart and brain ischaemia. FEBS J. 2013, 280, 4999–5014. [Google Scholar] [CrossRef] [PubMed]
- Daugas, E.; Susin, S.A.; Zamzami, N.; Ferri, K.F.; Irinopoulou, T.; Larochette, N.; Prévost, M.C.; Leber, B.; Andrews, D.; Penninger, J.; et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB. J. 2000, 14, 729–739. [Google Scholar] [PubMed]
- García-Heredia, J.M.; Díaz-Quintana, A.; Salzano, M.; Orzáez, M.; Pérez-Payá, E.; Teixeira, M.; de la Rosa, M.A.; Díaz-Moreno, I. Tyrosine phosphorylation turns alkaline transition into a biologically relevant process and makes human cytochrome c behave as an anti-apoptotic switch. J. Biol. Inorg. Chem. 2011, 16, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Jäättelä, M.; Wissing, D.; Kokholm, K.; Kallunki, T.; Egeblad, M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998, 17, 6124–6134. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, S.; Pandey, P.; Schofield, L.; Israels, S.; Roncinske, R.; Yoshida, K.; Bharti, A.; Yuan, Z.M.; Saxena, S.; Weichselbaum, R.; et al. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage-induced apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 6939–6942. [Google Scholar] [CrossRef] [PubMed]
- Brassai, A.; Suvanjeiev, R.G.; Bán, E.G.; Lakatos, M. Role of synaptic and nonsynaptic glutamate receptors in ischaemia induced neurotoxicity. Brain. Res. Bull. 2015, 112, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Cooper, C.E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994, 35, 295–298. [Google Scholar] [CrossRef]
- Borutaite, V.; Morkuniene, R.; Arandarcikaite, O.; Jekabsone, A.; Barauskaite, J.; Brown, G.C. Nitric oxide protects the heart from ischemia-induced apoptosis and mitochondrial damage via protein kinase G mediated blockage of permeability transition and cytochrome c release. J. Biomed. Sci. 2009, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Post, H.; Michel, M.C.; Kelm, M.; Schulz, R. Endogenous nitric oxide and myocardial adaptation to ischemia. Circ. Res. 2000, 87, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Isabelle, M.; Moreel, X.; Gagné, J.P.; Rouleau, M.; Ethier, C.; Gagné, P.; Hendzel, M.J.; Poirier, G.G. Investigation of PARP-1, PARP-2, and PARG interactomes by affinity-purification mass spectrometry. Proteome Sci. 2010, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.A.; Umanah, G.K.; Chang, C.; Stevens, D.A.; Karuppagounder, S.S.; Gagné, J.P.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 10209–10214. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.D.; Liu, G.L.; Wang, J.; Wang, H.; Zhang, J.N.; Zhang, F.; Ma, Y.; Ji, X.Y.; Li, C.; Zhang, M.X. Poly(ADP-ribose) polymerase 1 inhibition protects cardiomyocytes from inflammation and apoptosis in diabetic cardiomyopathy. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, M.J.; Sampei, K.; Mandir, A.S.; Hurn, P.D.; Traystman, R.J.; Bao, J.; Pieper, A.; Wang, Z.Q.; Dawson, T.M.; Snyder, S.H.; et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat. Med. 1997, 3, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Ogata, Y.; Suzuki, K.; Enghild, J.J.; Salvesen, G. Substrate specificities and activation mechanisms of matrix metalloproteinases. Biochem. Soc. Trans. 1991, 19, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hafez, S.; Abdelsaid, M.; El-Shafey, S.; Johnson, M.H.; Fagan, S.C.; Ergul, A. Matrix metalloprotease 3 exacerbates hemorrhagic transformation and worsens functional outcomes in hyperglycemic stroke. Stroke 2016, 47, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Chelluboina, B.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. Matrix metalloproteinase-12 induces blood-brain barrier damage after focal cerebral ischemia. Stroke 2015, 46, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front. Cell. Neurosci. 2016, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Kotlinska-Hasiec, E.; Czajkowski, M.; Rzecki, Z.; Stadnik, A.; Olszewski, K.; Rybojad, B.; Dabrowski, W. Disturbance in venous outflow from the cerebral circulation intensifies the release of blood-brain barrier injury biomarkers in patients undergoing cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Cooley, D.A.; DeBakey, M.E. Surgical considerations of intrathoracic aneurysms. Ann. Surg. 1952, 135, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Ergin, M.A.; Galla, J.D.; Lansman, S.L.; Quintana, C.; Bodian, C.; Griepp, R.B. Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J. Thorac. Cardiovasc. Surg. 1994, 107, 788–797. [Google Scholar] [PubMed]
- Ullery, B.W.; Wang, G.J.; Low, D.; Cheung, A.T. Neurological complications of thoracic endovascular aortic repair. Semin. Cardiothorac. Vasc. Anesth. 2011, 15, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Griepp, R.B.; di Luozzo, G. Hypothermia for aortic surgery. J. Thorac. Cardiovasc. Surg. 2013, 145, S56–S58. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.H.; Wan, B.; Bannon, P.G.; Misfeld, M.; LeMaire, S.A.; Kazui, T.; Kouchoukos, N.T.; Elefteriades, J.A.; Bavaria, J.; Coselli, J.S.; et al. A meta-analysis of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest with selective antegrade cerebral perfusion. Ann. Cardiothorac. Surg. 2013, 2, 148–158. [Google Scholar] [PubMed]
- Lima, B.; Williams, J.B.; Bhattacharya, S.D.; Shah, A.A.; Andersen, N.; Gaca, J.G.; Hughes, G.C. Results of proximal arch replacement using deep hypothermia for circulatory arrest: Is moderate hypothermia really justifiable? Am. Surg. 2011, 77, 1438–1444. [Google Scholar] [PubMed]
- Apostolakis, E.; Akinosoglou, K. The methodologies of hypothermic circulatory arrest and of antegrade and retrograde cerebral perfusion for aortic arch surgery. Ann. Thorac. Cardiovasc. Surg. 2008, 14, 138–148. [Google Scholar] [PubMed]
- Zhao, J.; Yang, J.; Liu, J.; Li, S.; Yan, J.; Meng, Y.; Wang, X.; Long, C. Effects of pulsatile and nonpulsatile perfusion on cerebral regional oxygen saturation and endothelin-1 in tetralogy of fallot infants. Artif. Organs 2011, 35, E54–E58. [Google Scholar] [CrossRef] [PubMed]
- Grubhofer, G.; Mares, P.; Rajek, A.; Müllner, T.; Haisjackl, M.; Dworschak, M.; Lassnigg, A. Pulsatility does not change cerebral oxygenation during cardiopulmonary bypass. Acta Anaesthesiol. Scand. 2000, 44, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.J.; van Oeveren, W.; Mungroop, H.E.; Epema, A.H.; den Hamer, I.J.; Keizer, J.J.; Leuvenink, R.P.; Mariani, M.A.; Rakhorst, G. Clinical effectiveness of centrifugal pump to produce pulsatile flow during cardiopulmonary bypass in patients undergoing cardiac surgery. Artif. Organs 2011, 35, E18–E26. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Kühne, L.; Grassl, M.; Gerdom, M.; von Salisch, S.; Vollroth, M.; Bakhtiary, F.; Mohr, F.W.; Dähnert, I.; Dhein, S. Protective effects of pulsatile flow during cardiopulmonary bypass. Ann. Thorac. Surg. 2015, 99, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Day, J.R.; Taylor, K.M. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int. J. Surg. 2005, 3, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Farsak, B.; Gunaydin, S.; Yildiz, U.; Sari, T.; Zorlutuna, Y. Clinical evaluation of leukocyte filtration as an alternative anti-inflammatory strategy to aprotinin in high-risk patients undergoing coronary revascularization. Surg. Today 2012, 42, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Bechtel, J.F.; Mühlenbein, S.; Eichler, W.; Marx, M.; Sievers, H.H. Leukocyte depletion during cardiopulmonary bypass in routine adult cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ganushchak, Y.M.; Fransen, E.J.; Visser, C.; de Jong, D.S.; Maessen, J.G. Neurological complications after coronary artery bypass grafting related to the performance of cardiopulmonary bypass. Chest 2004, 125, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; Dhein, S.; Ullmann, C.; Schneider, K.; Bilz, T.; Rastan, A.; Garbade, J.; Falk, V.; Emrich, F.C.; Muth, P.; et al. Cerebral protection during controlled hypoperfusion in a piglet model: Comparison of moderate (25 °C) versus deep (18 °C) hypothermia at various flow rates using intraoperative measurements and ex vivo investigation. Thorac. Cardiovasc. Surg. 2013, 61, 546–552. [Google Scholar] [PubMed]
- Cook, D.J.; Proper, J.A.; Orszulak, T.A.; Daly, R.C.; Oliver, W.C., Jr. Effect of pump flow rate on cerebral blood flow during hypothermic cardiopulmonary bypass in adults. J. Cardiothorac. Vasc. Anesth. 1997, 11, 415–419. [Google Scholar] [CrossRef]
- Billett, H.H. Hemoglobin and Hematocrit. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; pp. 718–719. [Google Scholar]
- Del Felice, A.; Tessari, M.; Formaggio, E.; Menon, T.; Petrilli, G.; Gamba, G.; Scarati, S.; Masiero, S.; Bortolami, O.; Faggian, G. Hemoglobin concentration affects electroencephalogram during cardiopulmonary bypass: An indication for neuro-protective values. Artif. Organs 2016, 40, 169–175. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, A.; Vélez, H.; SoItero, E.; Magraner, M.; Bredy, R. Lowest safe hematocrit level on cardiopulmonary bypass in patients undergoing coronary artery bypass grafting. Bol. Asoc. Med. P. R. 2011, 103, 25–29. [Google Scholar] [PubMed]
- Jansen, N.J.; van Oeveren, W.; van Vliet, M.; Stoutenbeek, C.P.; Eysman, L.; Wildevuur, C.R. The role of different types of corticosteroids on the inflammatory mediators in cardiopulmonary bypass. Eur. J. Cardiothorac. Surg. 1991, 5, 211–217. [Google Scholar] [CrossRef]
- Bourbon, A.; Vionnet, M.; Leprince, P.; Vaissier, E.; Copeland, J.; McDonagh, P.; Debré, P.; Gandjbakhch, I. The effect of methylprednisolone treatment on the cardiopulmonary bypass-induced systemic inflammatory response. Eur. J. Cardiothorac. Surg. 2004, 26, 932–938. [Google Scholar] [CrossRef] [PubMed]
- El Azab, S.R.; Rosseel, P.M.; de Lange, J.J.; Groeneveld, A.B.; van Strik, R.; van Wijk, E.M.; Scheffer, G.J. Dexamethasone decreases the pro- to anti-inflammatory cytokine ratio during cardiac surgery. Br. J. Anaesth. 2002, 88, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, M.P.; Rassias, A.J.; Guyre, P.M.; Sanders, J.H.; Beach, M.; Pahl, J.; Watson, R.B.; Whalen, P.K.; Yeo, K.T.; Yeager, M.P. Glucocorticoid effects on the inflammatory and clinical responses to cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2002, 16, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Sobieski, M.A., II; Graham, J.D.; Pappas, P.S.; Tatooles, A.J.; Slaughter, M.S. Reducing the effects of the systemic inflammatory response to cardiopulmonary bypass: Can single dose steroids blunt systemic inflammatory response syndrome? ASAIO J. 2008, 54, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Bocsi, J.; Hänzka, M.C.; Osmancik, P.; Hambsch, J.; Dähnert, I.; Sack, U.; Bellinghausen, W.; Schneider, P.; Janoušek, J.; Kostelka, M.; et al. Modulation of the cellular and humoral immune response to pediatric open heart surgery by methylprednisolone. Cytom. B Clin. Cytom. 2011, 80, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.N.; Bailey, S.M.; Smith, P.L.; Taylor, K.M.; Oatridge, A.; Bydder, G.M. Brain swelling in first hour after coronary artery bypass surgery. Lancet 1993, 342, 586–587. [Google Scholar] [CrossRef]
- Ottens, T.H.; Hendrikse, J.; Slooter, A.J.; van Herwerden, L.A.; Dieleman, J.M.; van Dijk, D. Low incidence of early postoperative cerebral edema after coronary artery bypass grafting. J. Cardiothorac. Vasc. Anesth. 2015, 29, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.M.; Nierich, A.P.; Rosseel, P.M.; van der Maaten, J.M.; Hofland, J.; Diephuis, J.C.; Schepp, R.M.; Boer, C.; Moons, K.G.; van Herwerden, L.A.; et al. Intraoperative high-dose dexamethasone for cardiac surgery: A randomized controlled trial. JAMA 2012, 308, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Baki, E.D.; Aldemir, M.; Kokulu, S.; Koca, H.B.; Ela, Y.; Sıvacı, R.G.; Öztürk, N.K.; Emmiler, M.; Adalı, F.; Uzel, H. Comparison of the effects of desflurane and propofol anesthesia on the inflammatory response and s100β protein during coronary artery bypass grafting. Inflammation 2013, 36, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Sayed, S.; Idriss, N.K.; Sayyedf, H.G.; Ashry, A.A.; Rafatt, D.M.; Mohamed, A.O.; Blann, A.D. Effects of propofol and isoflurane on haemodynamics and the inflammatory response in cardiopulmonary bypass surgery. Br. J. Biomed. Sci. 2015, 72, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Durgut, K.; Hosgor, K.; Gormus, N.; Ozergin, U.; Solak, H. The cerebroprotective effects of pentoxifylline and aprotinin during cardiopulmonary bypass in dogs. Perfusion 2004, 19, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.A.; Ghatak, S.B. Pexelizumab and its role in the treatment of myocardial infarction and in coronary artery bypass graft surgery: A review. Recent Pat. Cardiovasc. Drug Discov. 2008, 3, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Purusram, G.; Wang, H.; Yuan, R.; Xie, W.; Gui, P.; Dong, N.; Yao, S. The efficacy of parecoxib on systemic inflammatory response associated with cardiopulmonary bypass during cardiac surgery. Br. J. Clin. Pharmacol. 2013, 75, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xue, Q.; Yan, F.; Li, L.; Liu, J.; Li, S.; Hu, S. Ulinastatin as a neuroprotective and anti-inflammatory agent in infant piglets model undergoing surgery on hypothermic low-flow cardiopulmonary bypass. Paediatr. Anaesth. 2013, 23, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lin, J.; Yang, Y.; Zhou, J.; Gong, L.N.; Qin, Z.; Du, L. Lack of efficacy of ulinastatin therapy during cardiopulmonary bypass surgery. Chin. Med. J. 2015, 128, 3138–3142. [Google Scholar] [PubMed]
- Bhutta, A.T.; Schmitz, M.L.; Swearingen, C.; James, L.P.; Wardbegnoche, W.L.; Lindquist, D.M.; Glasier, C.M.; Tuzcu, V.; Prodhan, P.; Dyamenahalli, U.; et al. Ketamine as a neuroprotective and anti-inflammatory agent in children undergoing surgery on cardiopulmonary bypass: A pilot randomized, double-blind, placebo-controlled trial. Pediatr. Crit. Care Med. 2012, 13, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Liu, H.; Liu, C.H.; Wu, B.; Chen, L.F.; Zhong, B.H.; Liu, K.L. Synthesis of the optical isomers of a new anticholinergic drug, penehyclidine hydrochloride (8018). Bioorg. Med. Chem. Lett. 2005, 15, 1979–1982. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.J.; Sun, Y.J.; Zhang, T.Z.; Zhou, J.; Diao, Y.G. Penehyclidine hydrochloride attenuates the cerebral injury in a rat model of cardiopulmonary bypass. Can. J. Physiol. Pharmacol. 2013, 91, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Einenkel, A.; Kühne, L.; Grassl, M.; von Salisch, S.; Kiefer, P.; Vollroth, M.; Dähnert, I.; Dhein, S. Hippocampal neuroprotection by minocycline and epigallo-catechin-3-gallate against cardiopulmonary bypass-associated injury. Brain Pathol. 2015, 25, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Drabek, T.; Janata, A.; Wilson, C.D.; Stezoski, J.; Janesko-Feldman, K.; Tisherman, S.A.; Foley, L.M.; Verrier, J.D.; Kochanek, P.M. Minocycline attenuates brain tissue levels of TNF-α produced by neurons after prolonged hypothermic cardiac arrest in rats. Resuscitation 2014, 85, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef] [PubMed]
- Checchia, P.A.; Bronicki, R.A.; Muenzer, J.T.; Dixon, D.; Raithel, S.; Gandhi, S.K.; Huddleston, C.B. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children—A randomized trial. J. Thorac. Cardiovasc. Surg. 2013, 146, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.M.; Worner, M.; Khan, J.S.; Matata, B.M. Inspired nitric oxide and modulation of oxidative stress during cardiac surgery. Curr. Drug. Saf. 2009, 4, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Kasper, B.; Salameh, A.; Krausch, M.; Kiefer, P.; Kostelka, M.; Mohr, F.W.; Dhein, S. Epigallocatechin gallate attenuates cardiopulmonary bypass-associated lung injury. J. Surg. Res. 2016, 201, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Funamoto, M.; Masumoto, H.; Takaori, K.; Taki, T.; Setozaki, S.; Yamazaki, K.; Minakata, K.; Ikeda, T.; Hyon, S.H.; Sakata, R. Green tea polyphenol prevents diabetic rats from acute kidney injury after cardiopulmonary bypass. Ann. Thorac. Surg. 2016, 101, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Twal, M.; Kiefer, P.; Salameh, A.; Schnabel, J.; Ossmann, S.; von Salisch, S.; Krämer, K.; Sobiraj, A.; Kostelka, M.; Mohr, F.W.; et al. Reno-protective effects of epigallocatechingallate in a small piglet model of extracorporeal circulation. Pharmacol. Res. 2013, 67, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Morgan, A.P.; Kemp, H.G.; Sullivan, J.M.; Herrera, M.G.; Cahill, G.F., Jr. Brain metabolism during fasting. J. Clin. Investig. 1967, 46, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Suzuki, M.; Kitamura, Y.; Mori, S.; Sato, K.; Dohi, S.; Sato, T.; Matsuura, A.; Hiraide, A. β-Hydroxybutyrate, a cerebral function improving agent, protects rat brain against ischemic damage caused by permanent and transient focal cerebral ischemia. Jpn. J. Pharmacol. 2002, 89, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Tian, W.; Wei, T.; Liu, F. The neuroprotective effects of β-hydroxybutyrate on Aβ-injected rat hippocampus in vivo and in Aβ-treated PC-12 cells in vitro. Free Radic. Res. 2015, 49, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Heal, D.J.; Martin, K.F. KTX 0101: A potential metabolic approach to cytoprotection in major surgery and neurological disorders. CNS Drug Rev. 2005, 11, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Debska, G.; May, R.; Kicińska, A.; Szewczyk, A.; Elger, C.E.; Kunz, W.S. Potassium channel openers depolarize hippocampal mitochondria. Brain Res. 2001, 892, 42–50. [Google Scholar] [CrossRef]
- De Arriba, S.G.; Franke, H.; Pissarek, M.; Nieber, K.; Illes, P. Neuroprotection by ATP-dependent potassium channels in rat neocortical brain slices during hypoxia. Neurosci. Lett. 1999, 273, 13–16. [Google Scholar] [CrossRef]
- Shake, J.G.; Peck, E.A.; Marban, E.; Gott, V.L.; Johnston, M.V.; Troncoso, J.C.; Redmond, J.M.; Baumgartner, W.A. Pharmacologically induced preconditioning with diazoxide: A novel approach to brain protection. Ann. Thorac. Surg. 2001, 72, 1849–1854. [Google Scholar] [CrossRef]
- Hong, J.H.; Lee, H.; Lee, S.R. Protective effect of resveratrol against neuronal damage following transient global cerebral ischemia in mice. J. Nutr. Biochem. 2016, 27, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.M.; Sun, C.K.; Lin, Y.C.; Chang, L.T.; Kao, Y.H.; Yen, C.H.; Chen, Y.L.; Tsai, T.H.; Chua, S.; Shao, P.L.; et al. Combination of cyclosporine and erythropoietin improves brain infarct size and neurological function in rats after ischemic stroke. J. Transl. Med. 2011, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Sulemanji, D.S.; Dönmez, A.; Aldemir, D.; Sezgin, A.; Türkoglu, S. Dexmedetomidine during coronary artery bypass grafting surgery: Is it neuroprotective?—A preliminary study. Acta Anaesthesiol. Scand. 2007, 51, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Lakic, N.; Surlan, K.; Jerin, A.; Meglic, B.; Curk, N.; Bunc, M. Importance of erythropoietin in brain protection after cardiac surgery: A pilot study. Heart Surg. Forum 2010, 13, E185–E189. [Google Scholar] [CrossRef] [PubMed]
- Ehrenreich, H.; Weissenborn, K.; Prange, H.; Schneider, D.; Weimar, C.; Wartenberg, K.; Schellinger, P.D.; Bohn, M.; Becker, H.; Wegrzyn, M.; et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 2009, 40, e647–e656. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Ishida, K. Neuroprotection after major cardiovascular surgery. Curr. Treat. Opt. Neurol. 2015, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Rimpiläinen, J.; Pokela, M.; Kiviluoma, K.; Vainionpää, V.; Hirvonen, J.; Ohtonen, P.; Jäntti, V.; Anttila, V.; Heinonen, H.; Juvonen, T. The N-methyl-d-aspartate antagonist memantine has no neuroprotective effect during hypothermic circulatory arrest: A study in the chronic porcine model. J. Thorac. Cardiovasc. Surg. 2001, 121, 957–968. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salameh, A.; Dhein, S.; Dähnert, I.; Klein, N. Neuroprotective Strategies during Cardiac Surgery with Cardiopulmonary Bypass. Int. J. Mol. Sci. 2016, 17, 1945. https://doi.org/10.3390/ijms17111945
Salameh A, Dhein S, Dähnert I, Klein N. Neuroprotective Strategies during Cardiac Surgery with Cardiopulmonary Bypass. International Journal of Molecular Sciences. 2016; 17(11):1945. https://doi.org/10.3390/ijms17111945
Chicago/Turabian StyleSalameh, Aida, Stefan Dhein, Ingo Dähnert, and Norbert Klein. 2016. "Neuroprotective Strategies during Cardiac Surgery with Cardiopulmonary Bypass" International Journal of Molecular Sciences 17, no. 11: 1945. https://doi.org/10.3390/ijms17111945
APA StyleSalameh, A., Dhein, S., Dähnert, I., & Klein, N. (2016). Neuroprotective Strategies during Cardiac Surgery with Cardiopulmonary Bypass. International Journal of Molecular Sciences, 17(11), 1945. https://doi.org/10.3390/ijms17111945