Hinokitiol Inhibits Melanogenesis via AKT/mTOR Signaling in B16F10 Mouse Melanoma Cells
Abstract
:1. Introduction
2. Results
2.1. Anti-Melanogenic Activity of Hinokitiol in Vitro
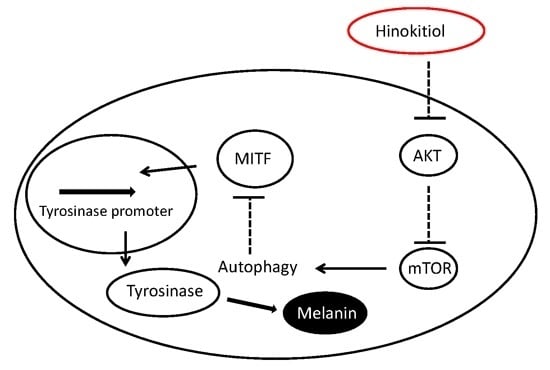
2.2. Hinokitiol Reduced Tyrosinase and MITF Expression through the Phosphorylation-Protein Kinase B (P-AKT)/Phosphorylation-Mammalian Target of the Rapamycin (P-mTOR) Pathway
2.3. Hinokitiol Reduced MITF via Inhibiting the Phosphorylation-AKT Signaling Pathway
3. Discussion
4. Experimental Section
4.1. Cells, Drugs and Plasmid
4.2. Cell Proliferation Assay
4.3. Evaluation of Melanin Content and Tyrosinase Activity
4.4. Western Blot Analysis
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ito, S.; Nakanishi, Y.; Valenzuela, R.K.; Brilliant, M.H.; Kolbe, L.; Wakamatsu, K. Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and phenomelanin in various tissue sample: Application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res. 2011, 24, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Park, S.H.; Kwon, S.B.; Li, K.; Youn, S.W.; Park, K.C. (−)-Epigallocatechin-3-gallate and hinokitiol reduce melanin synthesis via decreased MITF production. Arch. Pharm. Res. 2004, 27, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.G.; Bae, E.J.; Kim, D.S.; Park, S.H.; Kwon, S.B.; Na, J.I.; Park, K.C. Differential regulation of melanosomal proteins after hinokitiol treatment. J. Dermatol. Sci. 2006, 43, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Qiu, L.; Zhou, J.J.; Guo, H.Y.; Hu, Y.H.; Li, Z.C.; Wang, Q.; Chen, Q.X.; Liu, B. Inhibitory effects of hinokitiol on tyrosinase activity and melanin biosynthesis and its antimicrobial activities. J. Enzym. Inhib. Med. Chem. 2010, 25, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Chang, H.; Choi, H.; Shin, J.H.; Park, S.J.; Jo, Y.K.; Choi, E.S.; Baek, S.Y.; Kim, B.G.; Chang, J.W.; et al. Autophagy induced by resveratrol suppresses α-MSH-induced melanogenesis. Exp. Dermatol. 2014, 23, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Shin, J.H.; Seok, S.H.; Kim, J.B.; Chang, H.; Park, S.J.; Jo, Y.K.; Choi, E.S.; Park, J.S.; Yeom, M.H.; et al. Autophagy mediates anti-melanogenic activity of 3’-ODI in B16F1 melanoma cells. Biochem. Biophys. Res. Commun. 2013, 442, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Jo, Y.K.; Park, S.J.; Chang, H.; Shin, J.H.; Choi, E.S.; Kim, J.B.; Seok, S.H.; Kim, J.S.; Oh, J.S.; et al. ARP101 inhibits α-MSH-stimulated melanogenesis by regulation of autophagy in melanocytes. FEBS Lett. 2013, 587, 3955–3960. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.K.; Lin, S.T.; Chang, W.W.; Liu, L.W.; Li, T.Y.; Kuo, C.Y.; Hsieh, J.L.; Lee, C.H. Hinokitiol induces autophagy in murine breast and colorectal cancer cells. Environ. Toxicol. 2016, 31, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lin, S.T.; Liu, J.J.; Chang, W.W.; Hsieh, J.L.; Wang, W.K. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene Ther. 2014, 21, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Shiau, A.L.; Shen, Y.T.; Hsieh, J.L.; Wu, C.L.; Lee, C.H. Scutellaria barbata inhibits angiogenesis through downregulation of HIF-1 α in lung tumor. Environ. Toxicol. 2014, 29, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Pawelek, J. l-tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Brożyna, A.A.; Janjetovic, Z.; Brooks, D.L.; Schwab, L.P.; Skobowiat, C.; Jóźwicki, W.; Seagroves, T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014, 563, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell. Biol. 2012, 212, 1–115. [Google Scholar]
- Slominski, A.T.; Carlson, J.A. Melanoma resistance: A bright future for academicians and a challenge for patient advocates. Mayo Clin. Proc. 2014, 89, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Chang, W.W.; Kuan, Y.D.; Lin, S.T.; Hsu, H.C.; Lee, C.H. Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immun. 2012, 18, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.D.; Lee, C.H. Salmonella overcomes tumor immune tolerance by inhibition of tumor indoleamine 2, 3-dioxygenase 1 expression. Oncotarget 2016, 7, 374–385. [Google Scholar] [PubMed]
- Wang, W.K.; Chen, M.C.; Leong, H.F.; Kuo, Y.L.; Kuo, C.Y.; Lee, C.H. Connexin 43 suppresses tumor angiogenesis by down-regulation of vascular endothelial growth factor via hypoxic-induced factor-1α. Int. J. Mol. Sci. 2015, 16, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.S.; Kuan, Y.D.; Chiu, K.H.; Wang, W.K.; Chang, F.H.; Liu, C.H.; Lee, C.H. The extract of Rhodobacter sphaeroides inhibits melanogenesis through the MEK/ERK signaling pathway. Mar. Drugs 2013, 11, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.W.; Liu, J.J.; Liu, C.F.; Liu, W.S.; Lim, Y.P.; Cheng, Y.J.; Lee, C.H. An extract of Rhodobacter sphaeroides reduces cisplatin-induced nephrotoxicity in mice. Toxins 2013, 5, 2353–2365. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.K.; Lu, M.F.; Kuan, Y.D.; Lee, C.H. The treatment of mouse colorectal cancer by oral delivery tumor-targeting Salmonella. Am. J. Cancer. Res. 2015, 5, 2222–2228. [Google Scholar] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, Y.-T.; Huang, Y.-F.; Kuo, C.-Y.; Lin, Y.-C.; Chiang, W.-C.; Wang, W.-K.; Hsu, C.-W.; Lee, C.-H. Hinokitiol Inhibits Melanogenesis via AKT/mTOR Signaling in B16F10 Mouse Melanoma Cells. Int. J. Mol. Sci. 2016, 17, 248. https://doi.org/10.3390/ijms17020248
Tsao Y-T, Huang Y-F, Kuo C-Y, Lin Y-C, Chiang W-C, Wang W-K, Hsu C-W, Lee C-H. Hinokitiol Inhibits Melanogenesis via AKT/mTOR Signaling in B16F10 Mouse Melanoma Cells. International Journal of Molecular Sciences. 2016; 17(2):248. https://doi.org/10.3390/ijms17020248
Chicago/Turabian StyleTsao, Yu-Tzu, Yu-Fen Huang, Chun-Yu Kuo, Yu-Chiang Lin, Wei-Cheng Chiang, Wei-Kuang Wang, Chia-Wei Hsu, and Che-Hsin Lee. 2016. "Hinokitiol Inhibits Melanogenesis via AKT/mTOR Signaling in B16F10 Mouse Melanoma Cells" International Journal of Molecular Sciences 17, no. 2: 248. https://doi.org/10.3390/ijms17020248
APA StyleTsao, Y. -T., Huang, Y. -F., Kuo, C. -Y., Lin, Y. -C., Chiang, W. -C., Wang, W. -K., Hsu, C. -W., & Lee, C. -H. (2016). Hinokitiol Inhibits Melanogenesis via AKT/mTOR Signaling in B16F10 Mouse Melanoma Cells. International Journal of Molecular Sciences, 17(2), 248. https://doi.org/10.3390/ijms17020248