Integrative Analyses of miRNA-mRNA Interactions Reveal let-7b, miR-128 and MAPK Pathway Involvement in Muscle Mass Loss in Sex-Linked Dwarf Chickens
Abstract
:1. Introduction
2. Results
2.1. Muscle Fiber Characteristics in the SLD and Normal Chicken
2.2. Differentially Expressed Genes and miRNAs between SLD and Normal Chickens
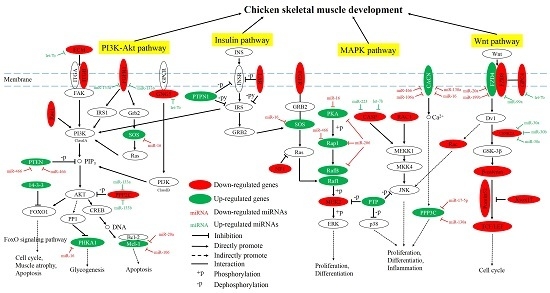
2.3. Network and Functional Analysis of the DEGs and DEMs at 7w
2.4. Differentially Expressed Genes and miRNAs between E14 and 7w
2.5. Functional and Network Analysis of the Strain-Specific Period DEGs and DEMs
2.6. Functional and Network Analysis of the Common DEGs and DEMs between E14 and 7w
3. Discussion
4. Experimental Section
4.1. Histology
4.2. Analyses of Microarray Data
4.3. Gene Ontology and miRNAs Target Pathway Analyses
4.4. miRNA-mRNA Interaction Analysis
4.5. miRNAs Quantitative Real-Time PCR (qPCR)
4.6. Western Blotting
4.7. Statistical Analysis
4.8. Ethics Standards
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, J.X.; Liu, Z.Z.; Yang, N. Deficiency of growth hormone receptor does not affect male reproduction in dwarf chickens. Poult. Sci. 2007, 86, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Xie, L.; Nie, Q.; Zeng, H.; Peng, Z.; Zhang, D.; Zhang, X. The effects of different sex-linked dwarf variations on Chinese native chickens. J. Integr. Agric. 2012, 11, 1500–1508. [Google Scholar] [CrossRef]
- Guillaume, J. The dwarfing gene dw: Its effects on anatomy, physiology, nutrition, management. Its application in poultry industry. World’s Poult. Sci. J. 1976, 32, 285–305. [Google Scholar] [CrossRef]
- Burnside, J.; Liou, S.S.; Zhong, C.; Cogburn, L.A. Abnormal growth hormone receptor gene expression in the sex-linked dwarf chicken. Gen. Comp. Endocrinol. 1992, 88, 20–28. [Google Scholar] [CrossRef]
- Dodgson, J.B.; Romanov, M.N. Use of chicken models for the analysis of human disease. Curr. Protoc. Hum. Genet. 2004, 15. [Google Scholar] [CrossRef]
- Godowski, P.J.; Leung, D.W.; Meacham, L.R.; Galgani, J.P.; Hellmiss, R.; Keret, R.; Rotwein, P.S.; Parks, J.S.; Laron, Z.; Wood, W.I. Characterization of the human growth hormone receptor gene and demonstration of a partial gene deletion in two patients with Laron-type dwarfism. Proc. Natl. Acad. Sci. USA 1989, 86, 8083–8087. [Google Scholar] [CrossRef] [PubMed]
- Eshet, R.; Laron, Z.; Pertzelan, A.; Arnon, R.; Dintzman, M. Defect of human growth hormone receptors in the liver of two patients with Laron-type dwarfism. Isr. J. Med. Sci. 1984, 20, 8–11. [Google Scholar] [PubMed]
- Berg, M.A.; Argente, J.; Chernausek, S.; Gracia, R.; Guevara-Aguirre, J.; Hopp, M.; Perez-Jurado, L.; Rosenbloom, A.; Toledo, S.P.; Francke, U. Diverse growth hormone receptor gene mutations in Laron syndrome. Am. J. Hum. Genet. 1993, 52, 998–1005. [Google Scholar] [PubMed]
- Gastier, J.M.; Berg, M.A.; Vesterhus, P.; Reiter, E.O.; Francke, U. Diverse deletions in the growth hormone receptor gene cause growth hormone insensitivity syndrome. Hum. Mutat. 2000, 16, 323–333. [Google Scholar] [CrossRef]
- Bass, S.H.; Mulkerrin, M.G.; Wells, J.A. A systematic mutational analysis of hormone-binding determinants in the human growth hormone receptor. Proc. Natl. Acad. Sci. USA 1991, 88, 4498–4502. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Baker, J.; Perkins, A.S.; Robertson, E.J.; Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993, 75, 59–72. [Google Scholar] [CrossRef]
- Coleman, M.E.; DeMayo, F.; Yin, K.C.; Lee, H.M.; Geske, R.; Montgomery, C.; Schwartz, R.J. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J. Biol. Chem. 1995, 270, 12109–12116. [Google Scholar] [CrossRef] [PubMed]
- Fiorotto, M.L.; Schwartz, R.J.; Delaughter, M.C. Persistent IGF-I overexpression in skeletal muscle transiently enhances DNA accretion and growth. FASEB J. 2003, 17, 59–60. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, A.J.; Stiles, A.D.; Underwood, L.E. Tissue concentrations of somatomedin C: Further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc. Natl. Acad. Sci. USA 1984, 81, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Gosteli-Peter, M.A.; Winterhalter, K.H.; Schmid, C.; Froesch, E.R.; Zapf, J. Expression and regulation of insulin-like growth factor-I (IGF-I) and IGF-binding protein messenger ribonucleic acid levels in tissues of hypophysectomized rats infused with IGF-I and growth hormone. Endocrinology 1994, 135, 2558–2567. [Google Scholar] [PubMed]
- Giustina, A.; Mazziotti, G.; Canalis, E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 2008, 29, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Junnila, R.K.; List, E.O.; Berryman, D.E.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Berryman, D.E.; Christiansen, J.S.; Johannsson, G.; Thorner, M.O.; Kopchick, J.J. Role of the GH/IGF-1 axis in lifespan and healthspan: Lessons from animal models. Growth Horm. IGF Res. 2008, 18, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Marzetti, E.; Borst, S.E.; Leeuwenburgh, C. Modulation of GH/IGF-1 axis: Potential strategies to counteract sarcopenia in older adults. Mech. Ageing Dev. 2008, 129, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Knizetova, H. Effects of the sex-linked dwarf gene (dw) on skeletal muscle cellularity in broiler chickens. Br. Poult. Sci. 1993, 34, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Y.; Tai, C.C.; Li, Y.H.; Wu, J.L. Progranulin compensates for blocked IGF-1 signaling to promote myotube hypertrophy in C2C12 myoblasts via the PI3K/Akt/mTOR pathway. FEBS Lett. 2012, 586, 3485–3492. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Adams, G.R. Inhibition of MAP/ERK kinase prevents IGF-I-induced hypertrophy in rat muscles. J. Appl. Physiol. 2004, 96, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Van Rooij, E.; Liu, N.; Olson, E.N. MicroRNAs flex their muscles. Trends Genet. 2008, 24, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Nie, Q.; Zhang, X. MicroRNAs involved in skeletal muscle differentiation. J. Genet. Genom. 2013, 40, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Ozata, D.M.; Caramuta, S.; Velazquez-Fernandez, D.; Akcakaya, P.; Xie, H.; Hoog, A.; Zedenius, J.; Backdahl, M.; Larsson, C.; Lui, W.O. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr. Relat. Cancer 2011, 18, 643–655. [Google Scholar] [CrossRef]
- Pallante, P.; Battista, S.; Pierantoni, G.M.; Fusco, A. Deregulation of microRNA expression in thyroid neoplasias. Nat. Rev. Endocrinol. 2014, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Tabruyn, S.P.; Hansen, S.; Ojeda-Fernandez, M.L.; Bovy, N.; Zarrabeitia, R.; Recio-Poveda, L.; Bernabeu, C.; Martial, J.A.; Botella, L.M.; Struman, I. MiR-205 is downregulated in hereditary hemorrhagic telangiectasia and impairs TGF-β signaling pathways in endothelial cells. Angiogenesis 2013, 16, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, H.; Mu, H.; Luo, W.; Li, Y.; Jia, X.; Wang, S.; Jia, X.; Nie, Q.; Li, Y.; et al. Let-7b regulates the expression of the growth hormone receptor gene in deletion-type dwarf chickens. BMC Genom. 2012, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, I.S.; Kostoulas, N.; Vergoulis, T.; Georgakilas, G.; Reczko, M.; Maragkakis, M.; Paraskevopoulou, M.D.; Prionidis, K.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA miRPath v.2.0: Investigating the combinatorial effect of microRNAs in pathways. Nucleic Acids Res. 2012, 40, W498–W504. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Cogburn, L.A.; Burnside, J. Comparison of gene expression in normal and growth hormone receptor-deficient dwarf chickens reveals a novel growth hormone regulated gene. Biochem. Biophys. Res. Commun. 1995, 206, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.E.; Lichanska, A.M.; Kerr, L.M.; White, M.; D’Aniello, E.M.; Maher, S.L.; Brown, R.; Teasdale, R.D.; Noakes, P.G.; Waters, M.J. In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol. Cell. Biol. 2005, 25, 66–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhart, B.J.; Slack, F.J.; Basson, M.; Pasquinelli, A.E.; Bettinger, J.C.; Rougvie, A.E.; Horvitz, H.R.; Ruvkun, G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 2000, 403, 901–906. [Google Scholar] [PubMed]
- Schultz, J.; Lorenz, P.; Gross, G.; Ibrahim, S.; Kunz, M. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res. 2008, 18, 549–557. [Google Scholar] [PubMed]
- Johnson, C.D.; Esquela-Kerscher, A.; Stefani, G.; Byrom, M.; Kelnar, K.; Ovcharenko, D.; Wilson, M.; Wang, X.; Shelton, J.; Shingara, J.; et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007, 67, 7713–7722. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.G.; Karam, R.; Huang, L.; Bhardwaj, A.; Lou, C.H.; Shum, E.Y.; Song, H.W.; Corbett, M.A.; Gifford, W.D.; Gecz, J.; et al. Identification of a microRNA that activates gene expression by repressing nonsense-mediated RNA decay. Mol. Cell 2011, 42, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Guo, P.; Lv, N.H.; Luo, L.Y. MiR-128, a key regulator of oncogenic properties. Mol. Biol. 2015, 49, 46–54. [Google Scholar] [CrossRef]
- Harding, R.L.; Velleman, S.G. MicroRNA regulation of myogenic satellite cell proliferation and differentiation. Mol. Cell. Biochem. 2016, 412, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhou, B.; Li, P.; Schinckel, A.P.; Liang, T.; Wang, H.; Li, H.; Fu, L.; Chu, Q.; Huang, R. MicroRNA-128 targets myostatin at coding domain sequence to regulate myoblasts in skeletal muscle development. Cell Signal. 2015, 27, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Keren, A.; Tamir, Y.; Bengal, E. The p38 MAPK signaling pathway: A major regulator of skeletal muscle development. Mol. Cell. Endocrinol. 2006, 252, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Kopchick, J.J.; Bellush, L.L.; Coschigano, K.T. Transgenic models of growth hormone action. Annu. Rev. Nutr. 1999, 19, 437–461. [Google Scholar] [CrossRef] [PubMed]
- Herrington, J.; Carter-Su, C. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol. Metab. 2001, 12, 252–257. [Google Scholar] [CrossRef]
- Landau, D.; Eshet, R.; Troib, A.; Gurman, Y.; Chen, Y.; Rabkin, R.; Segev, Y. Increased renal Akt/mTOR and MAPK signaling in type I diabetes in the absence of IGF type 1 receptor activation. Endocrine 2009, 36, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Zetser, A.; Gredinger, E.; Bengal, E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 1999, 274, 5193–5200. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, R.E. Signal transduction pathways that regulate eukaryotic protein synthesis. J. Biol. Chem. 1999, 274, 30337–30340. [Google Scholar] [CrossRef] [PubMed]
- Gredinger, E.; Gerber, A.N.; Tamir, Y.; Tapscott, S.J.; Bengal, E. Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. J. Biol. Chem. 1998, 273, 10436–10444. [Google Scholar] [CrossRef] [PubMed]
- Pallafacchina, G.; Blaauw, B.; Schiaffino, S. Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr. Metab. Cardiovasc. Dis. 2013, 23, S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Vasilatos-Younken, R.; Wang, X.H.; Zhou, Y.; Day, J.R.; McMurtry, J.P.; Rosebrough, R.W.; Decuypere, E.; Buys, N.; Darras, V.; Beard, J.L.; et al. New insights into the mechanism and actions of growth hormone (GH) in poultry. Domest. Anim. Endocrinol. 1999, 17, 181–190. [Google Scholar] [CrossRef]
- Halevy, O.; Hodik, V.; Mett, A. The effects of growth hormone on avian skeletal muscle satellite cell proliferation and differentiation. Gen. Comp. Endocrinol. 1996, 101, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zanou, N.; Gailly, P. Skeletal muscle hypertrophy and regeneration: Interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell. Mol. Life Sci. 2013, 70, 4117–4130. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.W.; Wright, A.E.; Mank, J.E. The evolution of gene expression and the transcriptome-phenotype relationship. Semin. Cell Dev. Biol. 2012, 23, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Brawand, D.; Soumillon, M.; Necsulea, A.; Julien, P.; Csardi, G.; Harrigan, P.; Weier, M.; Liechti, A.; Aximu-Petri, A.; Kircher, M.; et al. The evolution of gene expression levels in mammalian organs. Nature 2011, 478, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.F.; Goodyear, L.J. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J. Appl. Physiol. 2007, 103, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.; Perlman, H. Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 1997, 7, 597–602. [Google Scholar] [CrossRef]
- Walsh, K. Coordinate regulation of cell cycle and apoptosis during myogenesis. Prog. Cell Cycle Res. 1997, 3, 53–58. [Google Scholar] [PubMed]
- Zheng, Q.; Zhang, Y.; Chen, Y.; Yang, N.; Wang, X.J.; Zhu, D. Systematic identification of genes involved in divergent skeletal muscle growth rates of broiler and layer chickens. BMC Genom. 2009, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, R.; Zhang, Y.; Zhu, D. A systematic analysis of the skeletal muscle miRNA transcriptome of chicken varieties with divergent skeletal muscle growth identifies novel miRNAs and differentially expressed miRNAs. BMC Genom. 2011, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, Y.S.; Sivaprasad, U.; Malhotra, A.; Dutta, A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J. Cell Biol. 2006, 174, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.S.; Kawahara, G.; Motohashi, N.; Casar, J.C.; Eisenberg, I.; Myers, J.A.; Gasperini, M.J.; Estrella, E.A.; Kho, A.T.; Mitsuhashi, S.; et al. MicroRNA-199a is induced in dystrophic muscle and affects WNT signaling, cell proliferation, and myogenic differentiation. Cell Death Differ. 2013, 20, 1194–1208. [Google Scholar] [CrossRef] [PubMed]
- Naguibneva, I.; Ameyar-Zazoua, M.; Polesskaya, A.; Ait-Si-Ali, S.; Groisman, R.; Souidi, M.; Cuvellier, S.; Harel-Bellan, A. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat. Cell Biol. 2006, 8, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Foshay, K.M.; Gallicano, G.I. miR-17 family miRNAs are expressed during early mammalian development and regulate stem cell differentiation. Dev. Biol. 2009, 326, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; (South China Agricultural University, Guangzhou, China); Li, G.; (South China Agricultural University, Guangzhou, China). Personal communication, 2016.
- Luo, W.; Wu, H.; Ye, Y.; Li, Z.; Hao, S.; Kong, L.; Zheng, X.; Lin, S.; Nie, Q.; Zhang, X. The transient expression of miR-203 and its inhibiting effects on skeletal muscle cell proliferation and differentiation. Cell Death Dis. 2014, 5, e1347. [Google Scholar] [CrossRef] [PubMed]
- DAVID Bioinformatics Resources 6.7. Available online: http://david.abcc.ncifcrf.gov/home.jsp (accessed on 21 October 2015).
- DIANA TOOLS. Available online: http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=mirpath/index (accessed on 25 October 2015).
- MiRNA And Genes Integrated Analysis web tool. Available online: http://gencomp.bio.unipd.it/magia/start/ (accessed on 25 October 2015).
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.L.; Ideker, T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics 2011, 27, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Ingenuity IPA. Available online: http://www.ingenuity.com/products/ipa/ (accessed on 28 October 2015).









© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Lin, S.; Li, G.; Nie, Q.; Zhang, X. Integrative Analyses of miRNA-mRNA Interactions Reveal let-7b, miR-128 and MAPK Pathway Involvement in Muscle Mass Loss in Sex-Linked Dwarf Chickens. Int. J. Mol. Sci. 2016, 17, 276. https://doi.org/10.3390/ijms17030276
Luo W, Lin S, Li G, Nie Q, Zhang X. Integrative Analyses of miRNA-mRNA Interactions Reveal let-7b, miR-128 and MAPK Pathway Involvement in Muscle Mass Loss in Sex-Linked Dwarf Chickens. International Journal of Molecular Sciences. 2016; 17(3):276. https://doi.org/10.3390/ijms17030276
Chicago/Turabian StyleLuo, Wen, Shumao Lin, Guihuan Li, Qinghua Nie, and Xiquan Zhang. 2016. "Integrative Analyses of miRNA-mRNA Interactions Reveal let-7b, miR-128 and MAPK Pathway Involvement in Muscle Mass Loss in Sex-Linked Dwarf Chickens" International Journal of Molecular Sciences 17, no. 3: 276. https://doi.org/10.3390/ijms17030276
APA StyleLuo, W., Lin, S., Li, G., Nie, Q., & Zhang, X. (2016). Integrative Analyses of miRNA-mRNA Interactions Reveal let-7b, miR-128 and MAPK Pathway Involvement in Muscle Mass Loss in Sex-Linked Dwarf Chickens. International Journal of Molecular Sciences, 17(3), 276. https://doi.org/10.3390/ijms17030276