Impact of Lipoprotein Lipase Gene Polymorphism, S447X, on Postprandial Triacylglycerol and Glucose Response to Sequential Meal Ingestion
Abstract
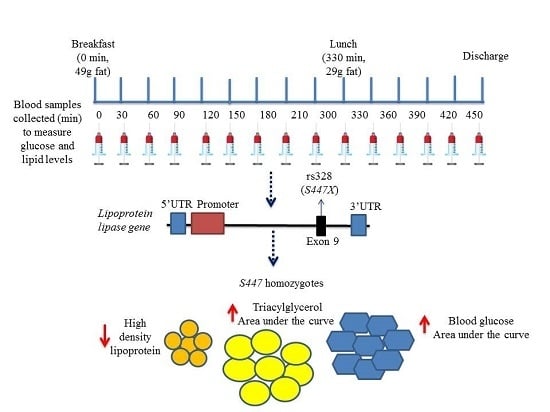
:1. Introduction
2. Results
3. Discussion
4. Experimental Section
4.1. Subjects
4.2. Sequential Test Meal Protocols
4.3. Biochemical Measurements
4.4. DNA Extraction and Genotyping
4.5. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Jackson, K.G.; Poppitt, S.D.; Minihane, A.M. Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 2012, 220, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, I.; Tverdal, A.; Urdal, P.; Graffiversen, S. Nonfasting serum triglyceride concentration and mortality from coronary heart-disease and any cause in middle-aged norwegian women. Br. Med. J. 1993, 307, 1318–1322. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Eckel, R.H.; Abumrad, N.A. Regulation of fatty acid uptake into tissues: Lipoprotein lipase- and CD36-mediated pathways. J. Lipid Res. 2009, 50, S86–S90. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.J. Lipoprotein lipase and lipolysis: Central roles in lipoprotein metabolism and atherogenesis. J. Lipid Res. 1996, 37, 693–707. [Google Scholar] [PubMed]
- Eriksson, J.W.; Buren, J.; Svensson, M.; Olivecrona, T.; Olivecrona, G. Postprandial regulation of blood lipids and adipose tissue lipoprotein lipase in type 2 diabetes patients and healthy control subjects. Atherosclerosis 2003, 166, 359–367. [Google Scholar] [CrossRef]
- Nierman, M.C.; Prinsen, B.H.; Rip, J.; Veldman, R.J.; Kuivenhoven, J.A.; Kastelein, J.J.; de Sain-van der Velden, M.G.; Stroes, E.S. Enhanced conversion of triglyceride-rich lipoproteins and increased low-density lipoprotein removal in LPLS447X carriers. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2410–2415. [Google Scholar] [CrossRef] [PubMed]
- Rip, J.; Nierman, M.C.; Ross, C.J.; Jukema, J.W.; Hayden, M.R.; Kastelein, J.J.; Stroes, E.S.; Kuivenhoven, J.A. Lipoprotein lipase S447X: A naturally occurring gain-of-function mutation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.; Babu, M.S.; Kaul, S.; Rajeshwar, K.; Balakrishna, N.; Jyothy, A. Association of LPL gene variant and LDL, HDL, VLDL cholesterol and triglyceride levels with ischemic stroke and its subtypes. J. Neurol. Sci. 2012, 318, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Tanguturi, P.R.; Pullareddy, B.; Rama Krishna, B.S.; Murthy, D.K. Lipoprotein lipase gene HindIII polymorphism and risk of myocardial infarction in South Indian population. Indian Heart J. 2013, 65, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Sagoo, G.S.; Tatt, I.; Salanti, G.; Butterworth, A.S.; Sarwar, N.; van Maarle, M.; Jukema, J.W.; Wiman, B.; Kastelein, J.J.; Bennet, A.M.; et al. Seven lipoprotein lipase gene polymorphisms, lipid fractions, and coronary disease: A HuGE association review and meta-analysis. Am. J. Epidemiol. 2008, 168, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Benlian, P.; de Gennes, J.L.; Foubert, L.; Zhang, H.; Gagne, S.E.; Hayden, M. Premature atherosclerosis in patients with familial chylomicronemia caused by mutations in the lipoprotein lipase gene. N. Engl. J. Med. 1996, 335, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Gotoda, T.; Yamada, N.; Kawamura, M.; Kozaki, K.; Mori, N.; Ishibashi, S.; Shimano, H.; Takaku, F.; Yazaki, Y.; Furuichi, Y.; et al. Heterogeneous mutations in the human lipoprotein lipase gene in patients with familial lipoprotein lipase deficiency. J. Clin. Investig. 1991, 88, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Miranda, J.; Cruz, G.; Gomez, P.; Marin, C.; Paz, E.; Perez-Martinez, P.; Fuentes, F.J.; Ordovas, J.M.; Perez-Jimenez, F. The influence of lipoprotein lipase gene variation on postprandial lipoprotein metabolism. J. Clin. Endocrinol. Metab. 2004, 89, 4721–4728. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulou, K.K.; Kolovou, G.D.; Kostakou, P.M.; Mihas, C.; Hatzigeorgiou, G.; Marvaki, C.; Degiannis, D.; Mikhailidis, D.P.; Cokkinos, D.V. Sex-associated effect of CETP and LPL polymorphisms on postprandial lipids in familial hypercholesterolaemia. Lipids Health Dis. 2009, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Humphries, S.E.; Nicaud, V.; Margalef, J.; Tiret, L.; Talmud, P.J. Lipoprotein lipase gene variation is associated with a paternal history of premature coronary artery disease and fasting and postprandial plasma triglycerides: The European Atherosclerosis Research Study (EARS). Arterioscler. Thromb. Vasc. Biol. 1998, 18, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Olano-Martin, E.; Abraham, E.C.; Gill-Garrison, R.; Valdes, A.M.; Grimaldi, K.; Tang, F.; Jackson, K.G.; Williams, C.M.; Minihane, A.M. Influence of apoA-V gene variants on postprandial triglyceride metabolism: Impact of gender. J. Lipid Res. 2008, 49, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Merkel, M.; Eckel, R.H.; Goldberg, I.J. Lipoprotein lipase: Genetics, lipid uptake, and regulation. J. Lipid Res. 2002, 43, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Myllykangas, L.; Polvikoski, T.; Sulkava, R.; Notkola, I.L.; Rastas, S.; Verkkoniemi, A.; Tienari, P.J.; Niinisto, L.; Hardy, J.; Perez-Tur, J.; et al. Association of lipoprotein lipase Ser447Ter polymorphism with brain infarction: A population-based neuropathological study. Ann. Med. 2001, 33, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Talmud, P.J.; Flavell, D.M.; Alfakih, K.; Cooper, J.A.; Balmforth, A.J.; Sivananthan, M.; Montgomery, H.E.; Hall, A.S.; Humphries, S.E. The lipoprotein lipase gene serine 447 stop variant influences hypertension-induced left ventricular hypertrophy and risk of coronary heart disease. Clin. Sci. (Lond.) 2007, 112, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, K.; Gotoda, T.; Kawamura, M.; Shimano, H.; Yazaki, Y.; Ouchi, Y.; Orimo, H.; Yamada, N. Mutational analysis of human lipoprotein lipase by carboxy-terminal truncation. J. Lipid Res. 1993, 34, 1765–1772. [Google Scholar] [PubMed]
- Turlo, K.; Leung, C.S.; Seo, J.J.; Goulbourne, C.N.; Adeyo, O.; Gin, P.; Voss, C.; Bensadoun, A.; Fong, L.G.; Young, S.G.; et al. Equivalent binding of wild-type lipoprotein lipase (LPL) and S447X-LPL to GPIHBP1, the endothelial cell LPL transporter. Biochim. Biophys. Acta 2014, 1841, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Onat, A.; Hergenc, G.; Agirbasli, M.; Kaya, Z.; Can, G.; Unaltuna, N.E. Preheparin serum lipoprotein lipase mass interacts with gender, gene polymorphism and, positively, with smoking. Clin. Chem. Lab. Med. 2009, 47, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Henderson, H.; Gagne, S.E.; Clee, S.M.; Miao, L.; Liu, G.; Hayden, M.R. Common sequence variants of lipoprotein lipase: Standardized studies of in vitro expression and catalytic function. Biochim. Biophys. Acta 1996, 1302, 159–166. [Google Scholar] [CrossRef]
- Kaser, S.; Sandhofer, A.; Holzl, B.; Gander, R.; Ebenbichler, C.F.; Paulweber, B.; Patsch, J.R. Phospholipid and cholesteryl ester transfer are increased in lipoprotein lipase deficiency. J. Intern. Med. 2003, 253, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Heden, T.D.; Liu, Y.; Sims, L.J.; Whaley-Connell, A.T.; Chockalingam, A.; Dellsperger, K.C.; Kanaley, J.A. Meal frequency differentially alters postprandial triacylglycerol and insulin concentrations in obese women. Obesity 2013, 21, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Armstrong, C.L.; Tang, M.; Mattes, R.D.; Campbell, W.W. The influence of higher protein intake and greater eating frequency on appetite control in overweight and obese men. Obesity 2010, 18, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Peacock, R.E.; Hamsten, A.; Nilsson-Ehle, P.; Humphries, S.E. Associations between lipoprotein lipase gene polymorphisms and plasma correlations of lipids, lipoproteins and lipase activities in young myocardial infarction survivors and age-matched healthy individuals from Sweden. Atherosclerosis 1992, 97, 171–185. [Google Scholar] [CrossRef]
- Ukkola, O.; Garenc, C.; Perusse, L.; Bergeron, J.; Despres, J.P.; Rao, D.C.; Bouchard, C. Genetic variation at the lipoprotein lipase locus and plasma lipoprotein and insulin levels in the Quebec Family Study. Atherosclerosis 2001, 158, 199–206. [Google Scholar] [CrossRef]
- Mead, J.R.; Ramji, D.P. The pivotal role of lipoprotein lipase in atherosclerosis. Cardiovasc. Res. 2002, 55, 261–269. [Google Scholar] [CrossRef]
- Robins, S.J.; Rubins, H.B.; Faas, F.H.; Schaefer, E.J.; Elam, M.B.; Anderson, J.W.; Collins, D.; Grp, V.-H.S. Insulin resistance and cardiovascular events with low HDL cholesterol - The Veterans Affairs HDL Intervention Trial (VA-HIT). Diabetes Care 2003, 26, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Borggreve, S.E.; de Vries, R.; Dullaart, R.P. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: Role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur. J. Clin. Investig. 2003, 33, 1051–1069. [Google Scholar] [CrossRef]
- Carvalho-Wells, A.L.; Jackson, K.G.; Lockyer, S.; Lovegrove, J.A.; Minihane, A.M. APOE genotype influences triglyceride and C-reactive protein responses to altered dietary fat intake in UK adults. Am. J. Clin. Nutr. 2012, 96, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Minihane, A.M.; Li, Y.; Gill, R.; Lovegrove, J.A.; Williams, C.M.; Jackson, K.G. The APOB insertion/deletion polymorphism (rs17240441) influences postprandial lipaemia in healthy adults. Nutr. Metab. 2015, 12, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couillard, C.; Bergeron, N.; Bergeron, J.; Pascot, A.; Mauriege, P.; Tremblay, A.; Prud’homme, D.; Bouchard, C.; Despres, J.P. Metabolic heterogeneity underlying postprandial lipemia among men with low fasting high density lipoprotein cholesterol concentrations. J. Clin. Endocrinol. Metab. 2000, 85, 4575–4582. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.G.; Clarke, D.T.; Murray, P.; Lovegrove, J.A.; O’Malley, B.; Minihane, A.M.; Williams, C.M. Introduction to the DISRUPT postprandial database: Subjects, studies and methodologies. Genes Nutr. 2010, 5, 39–48. [Google Scholar] [CrossRef] [PubMed]

| Participant’s Characteristics | S/S (n = 213) | S/X (n = 48) | Passociation |
|---|---|---|---|
| Age (years) | 53 ± 11 | 52 ± 11 | 0.648 |
| Men/Women | 125/88 | 27/21 | – |
| BMI (kg/m2) | 26.3 ± 3.4 | 27.4 ± 3.2 | 0.015 |
| Baseline Characteristics | |||
| TC (mmol/L) | 5.78 ± 1.05 | 5.62 ± 1.00 | 0.285 |
| TAG (mmol/L) | 1.67 ± 0.90 | 1.48 ± 0.51 | 0.057 |
| HDL-C (mmol/L) | 1.29 ± 0.42 | 1.40 ± 0.33 | 0.015 |
| LDL-C (mmol/L) | 3.73 ± 1.02 | 3.53 ± 0.92 | 0.167 |
| Glucose(mmol/L) | 5.16 ± 0.66 | 5.14 ± 0.45 | 0.534 |
| Insulin (pmol/L) | 48.3 ± 31.2 | 50.3 ± 26.2 | 0.517 |
| NEFA (μmol/L) | 519 ± 184 | 477 ± 170 | 0.102 |
| HOMA-IR | 1.96 ± 1.40 | 1.99 ± 1.09 | 0.376 |
| Postprandial summary measures | |||
| TAG AUC (mmol/L × 480 min) | 1193 ± 593 | 1046 ± 429 | 0.037 |
| TAG IAUC (mmol/L × 480 min) | 353 ± 228 | 305 ± 210 | 0.149 |
| NEFA AUC mmol/L × 300 min | 153 ± 45 | 149 ± 32 | 0.410 |
| NEFA IAUC (mmol/L × 300 min) | 96 ± 39 | 100 ± 26 | 0.792 |
| Glucose AUC (mmol/L × 480 min) | 3114 ± 460 | 2850 ± 763 | 0.006 |
| Glucose IAUC (mmol/L × 480 min) | 595 ± 284 | 460 ± 238 | 0.042 |
| Insulin AUC (nmol/L × 480 min) | 139 ± 94 | 123 ± 38 | 0.858 |
| Insulin IAUC (nmol/L × 480 min) | 114 ± 89 | 100 ± 34 | 0.876 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shatwan, I.M.; Minihane, A.-M.; Williams, C.M.; Lovegrove, J.A.; Jackson, K.G.; Vimaleswaran, K.S. Impact of Lipoprotein Lipase Gene Polymorphism, S447X, on Postprandial Triacylglycerol and Glucose Response to Sequential Meal Ingestion. Int. J. Mol. Sci. 2016, 17, 397. https://doi.org/10.3390/ijms17030397
Shatwan IM, Minihane A-M, Williams CM, Lovegrove JA, Jackson KG, Vimaleswaran KS. Impact of Lipoprotein Lipase Gene Polymorphism, S447X, on Postprandial Triacylglycerol and Glucose Response to Sequential Meal Ingestion. International Journal of Molecular Sciences. 2016; 17(3):397. https://doi.org/10.3390/ijms17030397
Chicago/Turabian StyleShatwan, Israa M., Anne-Marie Minihane, Christine M. Williams, Julie A. Lovegrove, Kim G. Jackson, and Karani S. Vimaleswaran. 2016. "Impact of Lipoprotein Lipase Gene Polymorphism, S447X, on Postprandial Triacylglycerol and Glucose Response to Sequential Meal Ingestion" International Journal of Molecular Sciences 17, no. 3: 397. https://doi.org/10.3390/ijms17030397
APA StyleShatwan, I. M., Minihane, A. -M., Williams, C. M., Lovegrove, J. A., Jackson, K. G., & Vimaleswaran, K. S. (2016). Impact of Lipoprotein Lipase Gene Polymorphism, S447X, on Postprandial Triacylglycerol and Glucose Response to Sequential Meal Ingestion. International Journal of Molecular Sciences, 17(3), 397. https://doi.org/10.3390/ijms17030397