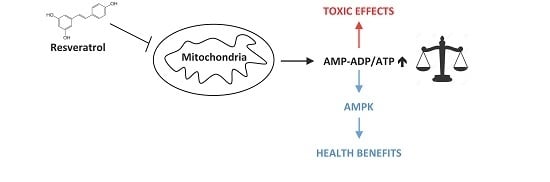
Resveratrol Inhibition of Cellular Respiration: New Paradigm for an Old Mechanism
Abstract
:1. Introduction
2. Biological Significance of Resveratrol in Plants
3. Molecular Mechanism of Resveratrol Toxicity
4. Relation between AMPK and Resveratrol
5. Amelioration of Chronic-Degenerative Diseases by Resveratrol
6. Induction of Antioxidant Systems by Resveratrol
7. Resveratrol and Mitochondrial Dysfunction
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| ADP | Adenosine diphosphate |
| AKT | Protein kinase B |
| AMP | Adenosine monophosphate |
| AMPK | AMP protein kinase |
| ApoB | Apolipoprotein B |
| CDD | Chronic-degenerative diseases |
| CRP | C-reactive protein |
| DR | Dietary restriction |
| EPAC1 | cAMP-regulated guanine nucleotide exchange factor |
| ETC | Electron transport chain |
| FAS | Fatty acid synthase |
| GST-P1 | Glutathione S-transferase pi 1 |
| HO-1 | Heme oxygenase-1 |
| IGF-1 | Insulin-like growth factor 1 |
| IKKα | lκB α kinase |
| IL-6 | Interleukine-6 |
| IRS1 | Insulin receptor substrate 1 |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| NASH | Non-alcoholic esteatohepatitis |
| Nrf2 | Nuclear factor-related factor 2 erythroid 2 |
| LDLc | Low-density lipoprotein |
| LDLox | LDL-oxidized |
| LKB1 | Liver kinase B1 |
| LXR | Liver X receptor |
| mTOR | Mechanistic target of rapamycin |
| NF-κB | Intranuclear nuclear factor-κB binding |
| NQO-1 | NAD(P)H quinone oxidoreductase 1 |
| PDE4 | cAMP phosphodiesterases |
| PGC1-α | Peroxisome proliferator-activated receptor γ coactivator |
| PI3K | Phosphatidylinositol 3-kinase |
| RSV | Resveratrol |
| RHEB | ras homolog enriched in brain |
| ROS | Reactive oxygen species |
| S6K1 | p70-S6 kinase 1 |
| SCD1 | Esterearoil CoA desaturase 1 |
| Sirt1 | Sirtuin 1 |
| SREBP-1c | Sterol regulatory element binding protein-1c |
| TNF-α | Tumor necrosis factor-α |
| TSC1/2 | Tuberous sclerosis complex 1/2 |
References
- Montero, C.; Cristescu, S.M.; Jimenez, J.B.; Orea, J.M.; te Lintel Hekkert, S.; Harren, F.J.; Gonzalez Urena, A. Trans-resveratrol and grape disease resistance. A dynamical study by high-resolution laser-based techniques. Plant Physiol. 2003, 131, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Dercks, W.; Creasy, L.L. The significance of stilbene phytoalexins in the plasmora viticola-grapevine interaction. Physiol. Mol. Plant Pathol. 1989, 34, 189–202. [Google Scholar] [CrossRef]
- Yang, M.H.; Kuo, C.H.; Hsieh, W.C.; Ku, K.L. Investigation of microbial elicitation of trans-resveratrol and trans-piceatannol in peanut callus led to the application of chitin as a potential elicitor. J. Agric. Food Chem. 2010, 58, 9537–9541. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Bessis, R.; Sbaghi, M.; Meunier, P. Production of the phytoalexin resveratrol by grapes as a response to botrytis attack under natural conditions. J. Phytopathol. 1995, 143, 135–139. [Google Scholar] [CrossRef]
- Barnes, R.A.; Gerber, N.N. The antifungal agent from osage orange wood. J. Am. Chem. Soc. 1955, 77, 3259–3262. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Evans, A.M.; et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of ampk activation. Cell Metab. 2010, 11, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, J.R.; Montgomery, M.G.; Leslie, A.G.; Walker, J.E. Mechanism of inhibition of bovine f1-atpase by resveratrol and related polyphenols. Proc. Natl. Acad. Sci. USA 2007, 104, 13632–13637. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting camp phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Labinskyy, N.; Mukhopadhyay, P.; Pinto, J.T.; Bagi, Z.; Ballabh, P.; Zhang, C.; Pacher, P.; Csiszar, A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1876–H1881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.Y.; Su, Y.; Gao, P.; Yang, Q.H.; Wang, Z.; Xu, Q. Resveratrol ameliorates high glucose-induced oxidative stress injury in human umbilical vein endothelial cells by activating ampk. Life Sci. 2015, 136, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Um, J.H.; Park, S.J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. Amp-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2010, 59, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Hebrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Nakabayashi, R.; Ogata, Y.; Sakurai, N.; Tokimatsu, T.; Goto, S.; Suzuki, M.; Jasinski, M.; Martinoia, E.; Otagaki, S.; et al. Multiomics in grape berry skin revealed specific induction of the stilbene synthetic pathway by ultraviolet-c irradiation. Plant Physiol. 2015, 168, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Hain, R.; Reif, H.J.; Krause, E.; Langebartels, R.; Kindl, H.; Vernau, B.; Weise, W.; Schmatzer, E.; Schreier, P.H. Disease resistance results from foreign phytoalexin expression in a novel plant. Nature 1993, 361, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Liswidowati; Melchior, F.; Hohmann, F.; Schwer, B.; Kindl, H. Induction of stilbene synthase by botrytis cinerea in cultured grapevine cells. Planta 1991, 183, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Wiese, W.; Vornam, B.; Krause, E.; Kindl, H. Structural organization and differential expression of three stilbene synthase genes located on a 13 kb grapevine DNA fragment. Plant Mol. Biol. 1994, 26. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by vitis vinifera and other members of the vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Ingham, J.L. Phytoalexins from the leguminosae. In Phytoalexins; Bailey, J.A., Mansfield, J.W., Eds.; Blackie: Glasgow/London, UK, 1982; pp. 21–80. [Google Scholar]
- Kuc, J. Phytoalexins, stress metabolism, and disease resisteance in plants. Ann. Rev. Phytopathol. 1995, 33, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Fornara, V.; Onelli, E.; Sparvoli, F.; Rossoni, M.; Aina, R.; Marino, G.; Citterio, S. Localization of stilbene synthase in vitis vinifera l. During berry development. Protoplasma 2008, 233, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Biological activity of resveratrol, a stilbenic compound from grapevines, against botrytis cinerea, the causal agent for gray mold. J. Chem. Ecol. 1997, 23, 1689–1702. [Google Scholar] [CrossRef]
- Vincenzi, S.; Tomasi, D.; Gaiotti, F.; Lovat, L.; Giacosa, S.; Torchio, F.; Río Segade, S.; Rolle, L. Comparative study of the resveratrol content of twenty-one italian red grape varieties. S. Afr. J. Enol. Vitic. 2013, 34, 30–35. [Google Scholar]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Selvaraj, S.; Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Dose-dependent interaction of trans-resveratrol with biomembranes: Effects on antioxidant property. J. Med. Chem. 2013, 56, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Brittes, J.; Lucio, M.; Nunes, C.; Lima, J.L.; Reis, S. Effects of resveratrol on membrane biophysical properties: Relevance for its pharmacological effects. Chem. Phys. Lipids 2010, 163, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Teskac, K.; Sinico, C.; Kristl, J. Effect of resveratrol incorporated in liposomes on proliferation and uv-b protection of cells. Int. J. Pharm. 2008, 363, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, J.; Micol, V.; de Godos, A.; Gomez-Fernandez, J.C. The cancer chemopreventive agent resveratrol is incorporated into model membranes and inhibits protein kinase c α activity. Arch. Biochem. Biophys. 1999, 372, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Slater, S.J.; Seiz, J.L.; Cook, A.C.; Stagliano, B.A.; Buzas, C.J. Inhibition of protein kinase c by resveratrol. Biochim. Biophys. Acta (BBA) 2003, 1637, 59–69. [Google Scholar] [CrossRef]
- Nimmanpisut, S.; Chudapongse, P.; Ratanabanangkoon, K. Effects of 2,4,3′,5′-tetrahydroxystilbene on oxidative phosphorylation by rat liver mitochondria. Biochem. Pharm. 1976, 25, 1245–1248. [Google Scholar] [CrossRef]
- Zini, R.; Morin, C.; Bertelli, A.; Bertelli, A.A.; Tillement, J. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp. Clin. Res. 1998, 25, 87–97. [Google Scholar]
- Zheng, J.; Ramirez, V.D. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 2000, 130, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Perez, L.A.; Nava, G.M.; Gonzalez-Hernandez, J.C.; Ramos-Gomez, M. Resveratrol increases glycolytic flux in saccharomyces cerevisiae via a SNF1-dependet mechanism. J. Bioenerg. Biomembr. 2015, 47, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Hughes, M.C.; Edgett, B.A.; Scribbans, T.D.; Simpson, C.A.; Perry, C.G.; Gurd, B.J. An examination of resveratrol’s mechanisms of action in human tissue: Impact of a single dose in vivo and dose responses in skeletal muscle ex vivo. PLoS ONE 2014, 9, e102406. [Google Scholar] [CrossRef] [PubMed]
- Dadi, P.K.; Ahmad, M.; Ahmad, Z. Inhibition of atpase activity of escherichia coli atp synthase by polyphenols. Int. J. Biol. Macromol. 2009, 45, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Higashida, K.; Kim, S.H.; Jung, S.R.; Asaka, M.; Holloszy, J.O.; Han, D.H. Effects of resveratrol and SIRT1 on pgc-1α activity and mitochondrial biogenesis: A reevaluation. PLoS Biol. 2013, 11, e1001603. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Kemp, B.E. Ampk in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Lage, R.; Dieguez, C.; Vidal-Puig, A.; Lopez, M. AMPK: A metabolic gauge regulating whole-body energy homeostasis. Trends Mol. Med. 2008, 14, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, B.P.; Gomes, A.P.; Dai, H.; Li, J.; Case, A.W.; Considine, T.; Riera, T.V.; Lee, J.E.; E, S.Y.; Lamming, D.W.; et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 2013, 339, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in amp-activated protein kinase activation. J. Biol. Chem. 2008, 283, 27628–27635. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef] [PubMed]
- Canto, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and sirt1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Sia, C.L.; Abuaysheh, S.; Korzeniewski, K.; Patnaik, P.; Marumganti, A.; Chaudhuri, A.; Dandona, P. An antiinflammatory and reactive oxygen species suppressive effects of an extract of polygonum cuspidatum containing resveratrol. J. Clin. Endocrinol. Metab. 2010, 95, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Sia, C.L.; Korzeniewski, K.; Lohano, T.; Abuaysheh, S.; Marumganti, A.; Chaudhuri, A.; Dandona, P. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J. Clin. Endocrinol. Metab. 2011, 96, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Sung, B.; Aggarwal, B.B. Age-associated chronic diseases require age-old medicine: Role of chronic inflammation. Prev. Med. 2012, 54 (Suppl.), S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Portillo, M.P.; Hijona, E.; Bujanda, L. Effects of resveratrol and other polyphenols in hepatic steatosis. WJG 2014, 20, 7366–7380. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, G. Isorhapontigenin and resveratrol suppress OXLDL-induced proliferation and activation of erk1/2 mitogen-activated protein kinases of bovine aortic smooth muscle cells. Biochem. Pharmacol. 2004, 67, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Garcia-Almagro, F.J.; Aviles-Plaza, F.; Parra, S.; Yanez-Gascon, M.J.; Ruiz-Ros, J.A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and APOB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Selective versus total insulin resistance: A pathogenic paradox. Cell Metab. 2008, 7, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. Mtor signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.S.; Findlay, G.M.; Gray, A.; Tolkacheva, T.; Wigfield, S.; Rebholz, H.; Barnett, J.; Leslie, N.R.; Cheng, S.; Shepherd, P.R.; et al. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J. Cell Biol. 2004, 166, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, P.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.; et al. Absence of s6k1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004, 431, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M. AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. World J. Gastroenterol. 2010, 16, 3731. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Yang, J.H.; Shin, B.Y.; Seo, K.; Shin, S.M.; Cho, I.J.; Ki, S.H. Resveratrol inhibits LXRα-dependent hepatic lipogenesis through novel antioxidant sestrin2 gene induction. Toxicol. Appl. Pharmacol. 2013, 271, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Kobayashi, M.; Kitagishi, Y. Roles for PI3K/AKT/PTEN pathway in cell signaling of nonalcoholic fatty liver disease. ISRN Endocrinol. 2013, 2013, 472432. [Google Scholar] [CrossRef] [PubMed]
- Duvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mtor complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.M.; Mieyal, J.J. Protein-thiol oxidation and cell death: Regulatory role of glutaredoxins. Antioxid. Redox Signal. 2012, 17, 1748–1763. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Cao, Z.; Goeddel, D.V. NF-κB inducing kinase activates IKK-α by phosphorylation of ser-176. Proc. Natl. Acad. Sci. USA 1998, 95, 3792–3797. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. Sirt1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Beeson, C.C.; Beeson, G.C.; Schnellmann, R.G. A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal. Biochem. 2010, 404, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Escote, X.; Miranda, M.; Menoyo, S.; Rodriguez-Porrata, B.; Carmona-Gutierrez, D.; Jungwirth, H.; Madeo, F.; Cordero, R.R.; Mas, A.; Tinahones, F.; et al. Resveratrol induces antioxidant defence via transcription factor YAP1P. Yeast 2012, 29, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.R.; Uddin, S.; Bu, R.; Khan, O.S.; Ahmed, S.O.; Ahmed, M.; Al-Kuraya, K.S. Resveratrol suppresses constitutive activation of AKT via generation of ros and induces apoptosis in diffuse large b cell lymphoma cell lines. PLoS ONE 2011, 6, e24703. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, M.; Penumathsa, S.V.; Koneru, S.; Juhasz, B.; Zhan, L.; Otani, H.; Bagchi, D.; Das, D.K.; Maulik, N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic. Biol. Med. 2007, 43, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Ingles, M.; Gambini, J.; Miguel, M.G.; Bonet-Costa, V.; Abdelaziz, K.M.; El Alami, M.; Vina, J.; Borras, C. Pten mediates the antioxidant effect of resveratrol at nutritionally relevant concentrations. BioMed Res. Int. 2014, 2014, 580852. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Human Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef] [PubMed]
- Camara, A.K.S.; Lesnefsky, E.J.; Stowe, D.F. Potential therapeutic benefits of strategies directed to mitochondria. Antioxid. Redox Signal. 2010, 13, 279–347. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rojo, C.; Rodriguez-Orozco, A.R. Importance of oxidative damage on the electron transport chain for the rational use of mitochondria-targeted antioxidants. Mini-Rev. Med. Chem. 2011, 11, 1–8. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two "hits"? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Forbes-Hernandez, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, W.; Wang, R.; Nan, Y.; Wang, Q.; Liu, W.; Jin, F. Resveratrol enhanced anticancer effects of cisplatin on non-small cell lung cancer cell lines by inducing mitochondrial dysfunction and cell apoptosis. Int. J. Oncol. 2015, 47, 1460–1468. [Google Scholar] [CrossRef] [PubMed]



| Protein | Mechanism of Activation or Inhibition | Consequences | References |
|---|---|---|---|
| Complex I–III | Resveratrol and DUQH2 could act competitively on complex III | Inhibition of ETC, increase of ROS production | [30] |
| F0F1-ATPase | Inhibition of the rotatory mechanism of the F1-ATPase | Decrease in ATP production, increase of ROS production and activation of intrinsic mitochondria-mediated apoptotic pathway | [7,31,35,36] |
| AMPK | The increase of AMP-ADP levels due to inhibition of the ETC and F0F1-ATPase by resveratrol, activates the gamma subunit of AMPK | Activation of catabolism: stimulation of energy production from glucose and fatty acids. Inhibition of the IRS1/PI3K/AKT pathway | [6,11] |
| mTOR | Activation of AMPK by resveratrol inhibits mTOR through TSC1/2 activation | Inhibition of anabolism allows counteracting insulin resistance, cholesterol accumulation and dyslipidemia | [11,52,54] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrigal-Perez, L.A.; Ramos-Gomez, M. Resveratrol Inhibition of Cellular Respiration: New Paradigm for an Old Mechanism. Int. J. Mol. Sci. 2016, 17, 368. https://doi.org/10.3390/ijms17030368
Madrigal-Perez LA, Ramos-Gomez M. Resveratrol Inhibition of Cellular Respiration: New Paradigm for an Old Mechanism. International Journal of Molecular Sciences. 2016; 17(3):368. https://doi.org/10.3390/ijms17030368
Chicago/Turabian StyleMadrigal-Perez, Luis Alberto, and Minerva Ramos-Gomez. 2016. "Resveratrol Inhibition of Cellular Respiration: New Paradigm for an Old Mechanism" International Journal of Molecular Sciences 17, no. 3: 368. https://doi.org/10.3390/ijms17030368
APA StyleMadrigal-Perez, L. A., & Ramos-Gomez, M. (2016). Resveratrol Inhibition of Cellular Respiration: New Paradigm for an Old Mechanism. International Journal of Molecular Sciences, 17(3), 368. https://doi.org/10.3390/ijms17030368