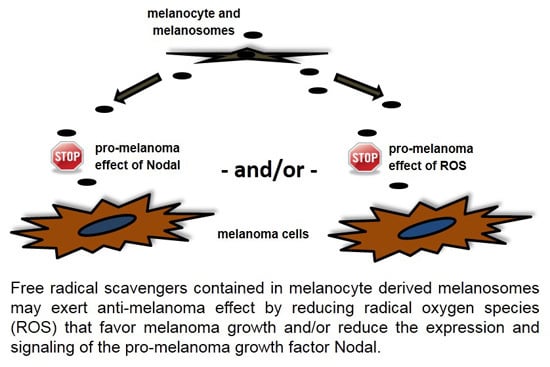
Melanocytes Affect Nodal Expression and Signaling in Melanoma Cells: A Lesson from Pediatric Large Congenital Melanocytic Nevi
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Patient Samples and Immunohistochemistry
3.2. Cell Cultures
3.3. Flow Cytometry
3.4. Cell Lysis and Western Blotting
3.5. Animal Experiments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Bandarchi, B.; Ma, L.; Navab, R.; Seth, A.; Rasty, G. From melanocyte to metastatic malignant melanoma. Dermatol. Res. Pract. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Borovansky, J.; Riley, P.A. Physiological and pathological functions of melanosomes. In Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological and Pathological Functions; Borovansky, J., Riley, P.A., Eds.; Wiley-Blackwell: Weinheim, Germany, 2011; pp. 343–381. [Google Scholar]
- Slominski, A. Neuroendocrine activity of the melanocyte. Exp. Dermatol. 2009, 18, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115. [Google Scholar]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. l-Tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Strizzi, L.; Hardy, K.M.; Kirschmann, D.A.; Ahrlund-Richter, L.; Hendrix, M.J. Nodal expression and detection in cancer: Experience and challenges. Cancer Res. 2012, 72, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.M. Nodal signaling: Developmental roles and regulation. Development 2007, 134, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Schier, A.F. Nodal signaling in vertebrate development. Annu. Rev. Cell Dev. Biol. 2003, 19, 589–621. [Google Scholar] [PubMed]
- Raya, A.; Kawakami, Y.; Rodriguez-Esteban, C.; Buscher, D.; Koth, C.M.; Itoh, T.; Morita, M.; Raya, R.M.; Dubova, I.; Bessa, J.G.; et al. Notch activity induces Nodal expression and mediates the establishment of left-right asymmetry in vertebrate embryos. Genes Dev. 2003, 17, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.M.; Kirschmann, D.A.; Seftor, E.A.; Margaryan, N.V.; Postovit, L.M.; Strizzi, L.; Hendrix, M.J. Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype. Cancer Res. 2010, 70, 10340–10350. [Google Scholar] [CrossRef] [PubMed]
- Strizzi, L.; Margaryan, N.V.; Gerami, P.; Haghighat, Z.; Harms, P.W.; Madonna, G.; Botti, G.; Ascierto, P.A.; Hendrix, M.J. Translational significance of Nodal, Cripto-1 and Notch4 in adult nevi. Oncol. Lett. 2016, in press. [Google Scholar]
- Castilla, E.E.; da Graça Dutra, M.; Orioli-Parreiras, I.M. Epidemiology of congenital pigmented naevi: I. Incidence rates and relative frequencies. Br. J. Dermatol. 1981, 104, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Arneja, J.S.; Gosain, A.K. Giant congenital melanocytic nevi. Plast. Reconstr. Surg. 2009, 124, 1e. [Google Scholar] [CrossRef] [PubMed]
- Donatien, P.; Surleve-Bazeille, J.E.; Thody, A.J.; Taieb, A. Growth and differentiation of normal human melanocytes in a TPA-free, cholera toxin-free, low-serum medium and influence of keratinocytes. Arch. Dermatol. Res. 1993, 285, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Kippenberger, S.; Bernd, A.; Bereiter-Hahn, J.; Ramirez-Bosca, A.; Kaufmann, R.; Holzmann, H. Transcription of melanogenesis enzymes in melanocytes: Dependence upon culture conditions and co-cultivation with keratinocytes. Pigment Cell Res. 1996, 9, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, J.J. The pigmentary system and inflammation. Pigment Cell Res. 1992, 5, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Seftor, E.A.; Brown, K.M.; Chin, L.; Kirschmann, D.A.; Wheaton, W.W.; Protopopov, A.; Feng, B.; Balagurunathan, Y.; Trent, J.M.; Nickoloff, B.J.; et al. Epigenetic transdifferentiation of normal melanocytes by a metastatic melanoma microenvironment. Cancer Res. 2005, 65, 10164–10169. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.M.; Strizzi, L.; Margaryan, N.V.; Gupta, K.; Murphy, G.F.; Scolyer, R.A.; Hendrix, M.J. Targeting Nodal in conjunction with dacarbazine induces synergistic anticancer effects in metastatic melanoma. Mol. Cancer Res. 2015, 13, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Strizzi, L.; Sandomenico, A.; Margaryan, N.V.; Focà, A.; Sanguigno, L.; Bodenstine, T.M.; Chandler, G.S.; Reed, D.W.; Gilgur, A.; Seftor, E.A.; et al. Effects of a novel Nodal-targeting monoclonal antibody in melanoma. Oncotarget 2015, 6, 34071–34086. [Google Scholar] [PubMed]
- Khalkhali-Ellis, Z.; Kirschmann, D.A.; Seftor, E.A.; Gilgur, A.; Bodenstine, T.M.; Hinck, A.P.; Hendrix, M.J. Divergence(s) in Nodal signaling between aggressive melanoma and embryonic stem cells. Int. J. Cancer 2015, 136, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Harms, P.W.; Pouryazdanparast, P.; Kim, D.S.; Ma, L.; Fullen, D.R. Expression of the embryonic morphogen Nodal in cutaneous melanocytic lesions. Mod. Pathol. 2010, 23, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Inácio, J.M.; Marques, S.; Nakamura, T.; Shinohara, K.; Meno, C.; Hamada, H.; Belo, J.A. The dynamic right-to-left translocation of Cerl2 is involved in the regulation and termination of Nodal activity in the mouse node. PLoS ONE 2013, 8, e60406. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lin, J.J.; Su, Z.Z.; Goldstein, N.I.; Fisher, P.B. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene 1995, 11, 2477–2486. [Google Scholar] [PubMed]
- Topczewska, J.M.; Postovit, L.M.; Margaryan, N.V.; Sam, A.; Hess, A.R.; Wheaton, W.W.; Nickoloff, B.J.; Topczewski, J.; Hendrix, M.J. Embryonic and tumorigenic pathways converge via Nodal signaling: Role in melanoma aggressiveness. Nat. Med. 2006, 12, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Carlson, J.A. Melanoma resistance: A bright future for academicians and a challenge for patient advocates. Mayo Clin. Proc. 2014, 89, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Wasmeier, C.; Hume, A.N.; Bolasco, G.; Seabra, M.C. Melanosomes at a glance. J. Cell Sci. 2008, 121, 3995–3999. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Niki, Y.; Yoshida, M.; Ito, M.; Akiyama, K.; Kim, J.H.; Yoon, T.J.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Involvement of pigment globules containing multiple melanosomes in the transfer of melanosomes from melanocytes to keratinocytes. Cell Logist 2011, 1, 12–20. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef] [PubMed]
- Cotter, M.A.; Thomas, J.; Cassidy, P.; Robinette, K.; Jenkins, N.; Florell, S.R.; Leachman, S.; Samlowski, W.E.; Grossman, D. N-Acetylcysteine protects melanocytes against oxidative stress/damage and delays onset of ultraviolet-induced melanoma in mice. Clin. Cancer Res. 2007, 13, 5952–5958. [Google Scholar] [CrossRef] [PubMed]
- Rózanowski, B.; Burke, J.M.; Boulton, M.E.; Sarna, T.; Rózanowska, M. Human RPE melanosomes protect from photosensitized and iron-mediated oxidation but become pro-oxidant in the presence of iron upon photodegradation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Taylor, M.J.; Walsh, L.A.; Dieters-Castator, D.; Das, P.; Jewer, M.; Zhang, G.; Postovit, L.M. Low oxygen levels induce the expression of the embryonic morphogen Nodal. Mol. Biol. Cell. 2011, 22, 4809–4821. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Takizawa, S.; van Ypersele de Strihou, C. Hypoxia. 1. Intracellular sensors for oxygen and oxidative stress: Novel therapeutic targets. Am. J. Physiol. Cell Physiol. 2011, 300, C226–C231. [Google Scholar] [CrossRef] [PubMed]
- Wittgen, H.G.; van Kempen, L.C. Reactive oxygen species in melanoma and its therapeutic implications. Melanoma Res. 2007, 17, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, B.B.; Satran, H.A.; Mork, S.J.; Margaryan, N.V.; Eide, G.E.; Petersen, K.; Strizzi, L.; Hendrix, M.J. Age-dependent association between protein expression of the embryonic stem cell marker Cripto-1 and survival of glioblastoma patients. Transl. Oncol. 2013, 6, 732–741. [Google Scholar]
- Lee, C.C.; Jan, H.J.; Lai, J.H.; Ma, H.I.; Hueng, D.Y.; Lee, Y.C.; Cheng, Y.Y.; Liu, L.W.; Wei, H.W.; Lee, H.M. Nodal promotes growth and invasion in human gliomas. Oncogene 2010, 29, 3110–3123. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margaryan, N.V.; Gilgur, A.; Seftor, E.A.; Purnell, C.; Arva, N.C.; Gosain, A.K.; Hendrix, M.J.C.; Strizzi, L. Melanocytes Affect Nodal Expression and Signaling in Melanoma Cells: A Lesson from Pediatric Large Congenital Melanocytic Nevi. Int. J. Mol. Sci. 2016, 17, 418. https://doi.org/10.3390/ijms17030418
Margaryan NV, Gilgur A, Seftor EA, Purnell C, Arva NC, Gosain AK, Hendrix MJC, Strizzi L. Melanocytes Affect Nodal Expression and Signaling in Melanoma Cells: A Lesson from Pediatric Large Congenital Melanocytic Nevi. International Journal of Molecular Sciences. 2016; 17(3):418. https://doi.org/10.3390/ijms17030418
Chicago/Turabian StyleMargaryan, Naira V., Alina Gilgur, Elisabeth A. Seftor, Chad Purnell, Nicoleta C. Arva, Arun K. Gosain, Mary J. C. Hendrix, and Luigi Strizzi. 2016. "Melanocytes Affect Nodal Expression and Signaling in Melanoma Cells: A Lesson from Pediatric Large Congenital Melanocytic Nevi" International Journal of Molecular Sciences 17, no. 3: 418. https://doi.org/10.3390/ijms17030418
APA StyleMargaryan, N. V., Gilgur, A., Seftor, E. A., Purnell, C., Arva, N. C., Gosain, A. K., Hendrix, M. J. C., & Strizzi, L. (2016). Melanocytes Affect Nodal Expression and Signaling in Melanoma Cells: A Lesson from Pediatric Large Congenital Melanocytic Nevi. International Journal of Molecular Sciences, 17(3), 418. https://doi.org/10.3390/ijms17030418