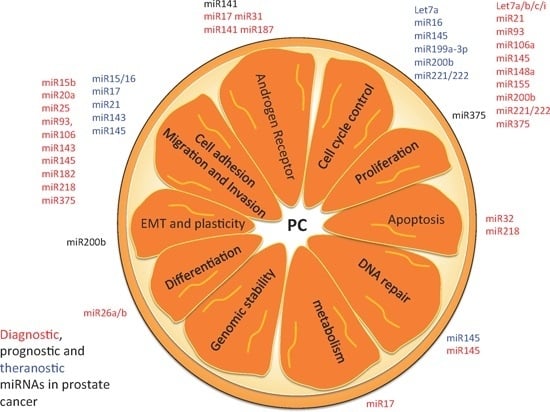
MicroRNAs as Biomarkers for Diagnosis, Prognosis and Theranostics in Prostate Cancer
Abstract
:1. Introduction
2. miRNA Biogenesis Altered in Prostate Cancer (PC)
3. miRNAs as Biomarkers of PC
3.1. Diagnostic miRNAs
3.1.1. Intracellular Diagnostic miRNAs
3.1.2. Extracellular Diagnostic miRNAs
3.2. Prognostic miRNAs
3.2.1. Intracellular Prognostic miRNAs
3.2.2. Extracellular Prognostic miRNAs
3.3. In-Silico Assessment of miRNA Signatures Found with Meta-Analysis
3.4. Pitfalls and Caveats of miRNA-Based Diagnostic and Prognostic Tools
4. Therapeutic miRNAs
5. Hallmarks of PC as Possible miRNA-Based Therapies
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PC | prostate cancer |
| miRs or miRNAs | microRNAs |
| N | normal tissue |
| T | tumor tissue |
References
- Johnson, L.M.; Choyke, P.L.; Figg, W.D.; Turkbey, B. The role of MRI in prostate cancer active surveillance. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhou, M. A concise update on prostate pathology. Ceskoslov. Patol. 2014, 50, 120–128. [Google Scholar]
- Humphrey, P.A. Gleason grading and prognostic factors in carcinoma of the prostate. Mod. Pathol. 2004, 17, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Brown, A.M.; Sankineni, S.; Wood, B.J.; Pinto, P.A.; Choyke, P.L. Multiparametric prostate magnetic resonance imaging in the evaluation of prostate cancer. CA Cancer J. Clin. 2015. [Google Scholar] [CrossRef] [PubMed]
- Vickers, J.; Thompson, A.; Collins, G.S.; Childs, M.; Hain, R. Place and provision of palliative care for children with progressive cancer: A study by the Paediatric Oncology Nurses’ Forum/United Kingdom Children’s Cancer Study Group Palliative Care Working Group. J. Clin. Oncol. 2007, 25, 4472–4476. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.B.; Partin, A.W.; Walsh, P.C.; Trock, B.J.; Veltri, R.W.; Nelson, W.G.; Coffey, D.S.; Singer, E.A.; Epstein, J.I. Gleason score 6 adenocarcinoma: Should it be labeled as cancer? J. Clin. Oncol. 2012, 30, 4294–4296. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Montorsi, F.; Catto, J.W. Future-proofing gleason grading: What to call gleason 6 prostate cancer? Eur. Urol. 2015, 68, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Pierorazio, P.M.; Walsh, P.C.; Partin, A.W.; Epstein, J.I. Prognostic gleason grade grouping: Data based on the modified gleason scoring system. BJU Int. 2013, 111, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Emberton, M. Will the attributes of multiparametric MRI permit the creation of a new approach to therapy? Curr. Opin. Urol. 2015, 25, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Ozen, M.; Creighton, C.J.; Ozdemir, M.; Ittmann, M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 2008, 27, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Porkka, K.P.; Pfeiffer, M.J.; Waltering, K.K.; Vessella, R.L.; Tammela, T.L.; Visakorpi, T. microRNA expression profiling in prostate cancer. Cancer Res. 2007, 67, 6130–6135. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.K.; Simms, M.S.; Maitland, N.J. Re: Yves Allorya, Willemien Beukers, Ana Sagrera, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: High frequency across stages, detection in urine, and lack of association with outcome. Eur urol 2014;65:360–6: Telomerase expression and stem cells: Urologic epithelial perspective. Eur. Urol. 2014, 65, e85–e86. [Google Scholar] [PubMed]
- Ren, Q.; Liang, J.; Wei, J.; Basturk, O.; Wang, J.; Daniels, G.; Gellert, L.L.; Li, Y.; Shen, Y.; Osman, I.; et al. Epithelial and stromal expression of miRNAs during prostate cancer progression. Am. J. Transl. Res. 2014, 6, 329–339. [Google Scholar] [PubMed]
- Lin, S.; Gregory, R.I. microRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, G.; Cava, C.; Castiglioni, I. microRNAs: New biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics 2015, 5, 1122–1143. [Google Scholar] [CrossRef] [PubMed]
- Ambs, S.; Prueitt, R.L.; Yi, M.; Hudson, R.S.; Howe, T.M.; Petrocca, F.; Wallace, T.A.; Liu, C.G.; Volinia, S.; Calin, G.A.; et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008, 68, 6162–6170. [Google Scholar] [CrossRef] [PubMed]
- Belair, C.D.; Paikari, A.; Moltzahn, F.; Shenoy, A.; Yau, C.; Dall’Era, M.; Simko, J.; Benz, C.; Blelloch, R. DGCR8 is essential for tumor progression following PTEN loss in the prostate. EMBO Rep. 2015, 16, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Chiosea, S.; Jelezcova, E.; Chandran, U.; Acquafondata, M.; McHale, T.; Sobol, R.W.; Dhir, R. Up-regulation of dicer, a component of the microRNA machinery, in prostate adenocarcinoma. Am. J. Pathol. 2006, 169, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal miRNAs as biomarkers for prostate cancer. Front. Genet. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Javidi, M.A.; Ahmadi, A.H.; Bakhshinejad, B.; Nouraee, N.; Babashah, S.; Sadeghizadeh, M. Cell-free microRNAs as cancer biomarkers: The odyssey of miRNAs through body fluids. Med. Oncol. 2014, 31. [Google Scholar] [CrossRef] [PubMed]
- Pigati, L.; Yaddanapudi, S.C.; Iyengar, R.; Kim, D.J.; Hearn, S.A.; Danforth, D.; Hastings, M.L.; Duelli, D.M. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS ONE 2010, 5, e13515. [Google Scholar] [CrossRef] [PubMed]
- Sita-Lumsden, A.; Dart, D.A.; Waxman, J.; Bevan, C.L. Circulating microRNAs as potential new biomarkers for prostate cancer. Br. J. Cancer 2013, 108, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.; Li, S.; Chin, A.R.; et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.; Hamar, P.; Guo, C.; Basar, E.; Perdigao-Henriques, R.; Balaj, L.; Lieberman, J. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Investig. 2014, 124, 5109–5128. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Hagiwara, K.; Takeshita, F.; Ochiya, T. Competitive interactions of cancer cells and normal cells via secretory microRNAs. J. Biol. Chem. 2012, 287, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Roa, W.; Brunet, B.; Guo, L.; Amanie, J.; Fairchild, A.; Gabos, Z.; Nijjar, T.; Scrimger, R.; Yee, D.; Xing, J. Identification of a new microRNA expression profile as a potential cancer screening tool. Clin. Investig. Med. Med. Clin. Exp. 2010, 33, E124–E132. [Google Scholar]
- Selth, L.A.; Roberts, M.J.; Chow, C.W.; Marshall, V.R.; Doi, S.A.; Vincent, A.D.; Butler, L.M.; Lavin, M.F.; Tilley, W.D.; Gardiner, R.A. Human seminal fluid as a source of prostate cancer-specific microRNA biomarkers. Endocr. Relat. Cancer 2014, 21, L17–L21. [Google Scholar] [CrossRef] [PubMed]
- Szczyrba, J.; Loprich, E.; Wach, S.; Jung, V.; Unteregger, G.; Barth, S.; Grobholz, R.; Wieland, W.; Stohr, R.; Hartmann, A.; et al. The microRNA profile of prostate carcinoma obtained by deep sequencing. Mol. Cancer Res. 2010, 8, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.; Jung, M.; Mollenkopf, H.J.; Wagner, I.; Stephan, C.; Jentzmik, F.; Miller, K.; Lein, M.; Kristiansen, G.; Jung, K. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int. J. Cancer 2010, 126, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Wach, S.; Nolte, E.; Szczyrba, J.; Stohr, R.; Hartmann, A.; Orntoft, T.; Dyrskjot, L.; Eltze, E.; Wieland, W.; Keck, B.; et al. MicroRNA profiles of prostate carcinoma detected by multiplatform microRNA screening. Int. J. Cancer 2012, 130, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.F.; Wu, J.T.; Wang, J.M.; Liu, Q.Z.; Gao, Z.L.; Liu, Y.X. MicroRNA expression profile analysis reveals diagnostic biomarker for human prostate cancer. Asian Pac. J. Cancer Prev. 2012, 13, 3313–3317. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.D.; Miller, N.; Sweeney, K.J.; Durkan, G.C.; Rogers, E.; Walsh, K.; Kerin, M.J. A circulating microRNA signature as a biomarker for prostate cancer in a high risk group. J. Clin. Med. 2015, 4, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Salas, I.; Rubio-Briones, J.; Calatrava, A.; Mancarella, C.; Masia, E.; Casanova, J.; Fernandez-Serra, A.; Rubio, L.; Ramirez-Backhaus, M.; Arminan, A.; et al. Identification of miR-187 and miR-182 as biomarkers of early diagnosis and prognosis in patients with prostate cancer treated with radical prostatectomy. J. Urol. 2014, 192, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Catto, J.W.; Shariat, S.F. The changing face of renal cell carcinoma: The impact of systematic genetic sequencing on our understanding of this tumor′s biology. Eur. Urol. 2013, 63, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Coppola, V.; de Maria, R.; Bonci, D. MicroRNAs and prostate cancer. Endocr. Relat. Cancer 2010, 17, F1–F17. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Phuyal, S.; Brech, A.; Sandvig, K.; Llorente, A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim. Biophys. Acta 2012, 1819, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Selth, L.A.; Townley, S.; Gillis, J.L.; Ochnik, A.M.; Murti, K.; Macfarlane, R.J.; Chi, K.N.; Marshall, V.R.; Tilley, W.D.; Butler, L.M. Discovery of circulating microRNAs associated with human prostate cancer using a mouse model of disease. Int. J. Cancer 2012, 131, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Lodes, M.J.; Caraballo, M.; Suciu, D.; Munro, S.; Kumar, A.; Anderson, B. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS ONE 2009, 4, e6229. [Google Scholar] [CrossRef] [PubMed]
- Moltzahn, F.; Olshen, A.B.; Baehner, L.; Peek, A.; Fong, L.; Stoppler, H.; Simko, J.; Hilton, J.F.; Carroll, P.; Blelloch, R. Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the sera of prostate cancer patients. Cancer Res. 2011, 71, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Yaman Agaoglu, F.; Kovancilar, M.; Dizdar, Y.; Darendeliler, E.; Holdenrieder, S.; Dalay, N.; Gezer, U. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumour Biol. 2011, 32, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Brase, J.C.; Johannes, M.; Schlomm, T.; Falth, M.; Haese, A.; Steuber, T.; Beissbarth, T.; Kuner, R.; Sultmann, H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer 2011, 128, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Mahn, R.; Heukamp, L.C.; Rogenhofer, S.; von Ruecker, A.; Muller, S.C.; Ellinger, J. Circulating microRNAs (miRNA) in serum of patients with prostate cancer. Urology 2011, 77, e1269–e1216. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.J.; Pawlowski, T.; Catto, J.W.; Marsden, G.; Vessella, R.L.; Rhees, B.; Kuslich, C.; Visakorpi, T.; Hamdy, F.C. Changes in circulating microRNA levels associated with prostate cancer. Br. J. Cancer 2012, 106, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Haldrup, C.; Kosaka, N.; Ochiya, T.; Borre, M.; Hoyer, S.; Orntoft, T.F.; Sorensen, K.D. Profiling of circulating microRNAs for prostate cancer biomarker discovery. Drug Deliv. Transl. Res. 2014, 4, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Zhang, G.L.; Li, H.R.; Luo, J.D.; Li, Z.X.; Chen, G.M.; Yang, J. A panel of five circulating microRNAs as potential biomarkers for prostate cancer. Prostate 2012, 72, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Medina-Villaamil, V.; Martinez-Breijo, S.; Portela-Pereira, P.; Quindos-Varela, M.; Santamarina-Cainzos, I.; Anton-Aparicio, L.M.; Gomez-Veiga, F. Circulating microRNAs in blood of patients with prostate cancer. Actas Urol. Esp. 2014, 38, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Kachakova, D.; Mitkova, A.; Popov, E.; Popov, I.; Vlahova, A.; Dikov, T.; Christova, S.; Mitev, V.; Slavov, C.; Kaneva, R. Combinations of serum prostate-specific antigen and plasma expression levels of let-7c, miR-30c, miR-141, and miR-375 as potential better diagnostic biomarkers for prostate cancer. DNA Cell Biol. 2015, 34, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Ahumada-Tamayo, S.; Saavedra-Briones, D.; Cantellano-Orozco, M.; Salido-Guadarrama, A.; Rodríguez-Dorantes, M.; Urdiales-Ortiz, A.; Hernández-Castellanos, V.; Merayo-Chalico, C.; Sánchez-Turati, G.; Santana-Ríos, Z.; et al. MicroRNA determination in urine for prostate cancer detection in Mexican patients at the hospital general “Dr. Manuelgea gonzález”. Rev. Mex. Urol. 2011, 71, 213–217. [Google Scholar]
- Egidi, M.G.; Cochetti, G.; Serva, M.R.; Guelfi, G.; Zampini, D.; Mechelli, L.; Mearini, E. Circulating microRNAs and kallikreins before and after radical prostatectomy: Are they really prostate cancer markers? BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.Y. P53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Ngezahayo, A.; Murua Escobar, H.; Nolte, I. Role of miRNA let-7 and its major targets in prostate cancer. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, M.; Coppola, V.; Addario, A.; Patrizii, M.; Maugeri-Sacca, M.; Memeo, L.; Colarossi, C.; Francescangeli, F.; Biffoni, M.; Collura, D.; et al. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene 2011, 30, 4231–4242. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, W.W.; Li, H.; Liu, F.; Khorshidi, A.; Rutnam, Z.J.; Yang, B.B. Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Nucleic Acids Res. 2013, 41, 9688–9704. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Lee, E.J.; Esau, C.; Schmittgen, T.D. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas 2009, 38, e190–e199. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Dai, W.Q.; Xu, X.F.; Wang, F.; He, L.; Guo, C.Y. Effects of multiple-target anti-microRNA antisense oligodeoxyribonucleotides on proliferation and migration of gastric cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 3203–3207. [Google Scholar] [CrossRef] [PubMed]
- Zoni, E.; van der Horst, G.; van de Merbel, A.F.; Chen, L.; Rane, J.K.; Pelger, R.C.; Collins, A.T.; Visakorpi, T.; Snaar-Jagalska, B.E.; Maitland, N.J.; et al. miR-25 modulates invasiveness and dissemination of human prostate cancer cells via regulation of αv- and α6-integrin expression. Cancer Res. 2015, 75, 2326–2336. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.W.; Fulgham, P.; Jay, C.; Chen, P.; Khalil, I.; Liu, S.; Senzer, N.; Eklund, A.C.; Han, J.; Nemunaitis, J. MicroRNA profile analysis of human prostate cancers. Cancer Gene Ther. 2009, 16, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Volinia, S.; Calin, G.A.; Liu, C.G.; Ambs, S.; Cimmino, A.; Petrocca, F.; Visone, R.; Iorio, M.; Roldo, C.; Ferracin, M.; et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA 2006, 103, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Leite, K.R.; Tomiyama, A.; Reis, S.T.; Sousa-Canavez, J.M.; Sanudo, A.; Dall’Oglio, M.F.; Camara-Lopes, L.H.; Srougi, M. MicroRNA-100 expression is independently related to biochemical recurrence of prostate cancer. J. Urol. 2011, 185, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Martens-Uzunova, E.S.; Jalava, S.E.; Dits, N.F.; van Leenders, G.J.; Moller, S.; Trapman, J.; Bangma, C.H.; Litman, T.; Visakorpi, T.; Jenster, G. Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene 2012, 31, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiyama, K.; Ito, H.; Taga, M.; Naganuma, S.; Oshinoya, Y.; Nagano, K.; Yokoyama, O.; Itoh, H. Expression of microRNAs associated with gleason grading system in prostate cancer: miR-182-5p is a useful marker for high grade prostate cancer. Prostate 2013, 73, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Lichner, Z.; Fendler, A.; Saleh, C.; Nasser, A.N.; Boles, D.; Al-Haddad, S.; Kupchak, P.; Dharsee, M.; Nuin, P.S.; Evans, K.R.; et al. MicroRNA signature helps distinguish early from late biochemical failure in prostate cancer. Clin. Chem. 2013, 59, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Spahn, M.; Kneitz, S.; Scholz, C.J.; Joniau, S.; Stroebel, P.; Riedmiller, H.; Kneitz, B. Distinct microRNA expression profile in prostate cancer patients with early clinical failure and the impact of let-7 as prognostic marker in high-risk prostate cancer. PLoS ONE 2013, 8, e65064. [Google Scholar] [CrossRef] [PubMed]
- Larne, O.; Martens-Uzunova, E.; Hagman, Z.; Edsjo, A.; Lippolis, G.; den Berg, M.S.; Bjartell, A.; Jenster, G.; Ceder, Y. miQ—A novel microRNA based diagnostic and prognostic tool for prostate cancer. Int. J. Cancer. 2013, 132, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Haflidadottir, B.S.; Larne, O.; Martin, M.; Persson, M.; Edsjo, A.; Bjartell, A.; Ceder, Y. Upregulation of miR-96 enhances cellular proliferation of prostate cancer cells through FOXO1. PLoS ONE 2013, 8, e72400. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinheiro, P.; Montezuma, D.; Henrique, R.; Jeronimo, C. Diagnostic and prognostic epigenetic biomarkers in cancer. Epigenomics 2015, 7, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.; Gordanpour, A.; Bendavid, J.S.; Sugar, L.; Nam, R.K.; Seth, A. miR-182 is associated with growth, migration and invasion in prostate cancer via suppression of FOXO1. J. Cancer 2015, 6, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, L.; Yi, X.; Yu, X. Diagnostic and prognostic values of tissue hsa-miR-30c and hsa-miR-203 in prostate carcinoma. Tumour Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Varambally, S.; Cao, Q.; Mani, R.S.; Shankar, S.; Wang, X.; Ateeq, B.; Laxman, B.; Cao, X.; Jing, X.; Ramnarayanan, K.; et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 2008, 322, 1695–1699. [Google Scholar]
- Cai, C.; Chen, Q.B.; Han, Z.D.; Zhang, Y.Q.; He, H.C.; Chen, J.H.; Chen, Y.R.; Yang, S.B.; Wu, Y.D.; Zeng, Y.R.; et al. miR-195 inhibits tumor progression by targeting RPS6KB1 in human prostate cancer. Clin. Cancer Res. 2015, 21, 4922–4934. [Google Scholar] [CrossRef] [PubMed]
- Dezhong, L.; Xiaoyi, Z.; Xianlian, L.; Hongyan, Z.; Guohua, Z.; Bo, S.; Shenglei, Z.; Lian, Z. miR-150 is a factor of survival in prostate cancer patients. J. BUON 2015, 20, 173–179. [Google Scholar] [PubMed]
- Zhang, H.; Qi, S.; Zhang, T.; Wang, A.; Liu, R.; Guo, J.; Wang, Y.; Xu, Y. miR-188-5p inhibits tumour growth and metastasis in prostate cancer by repressing LAPTM4B expression. Oncotarget 2015, 6, 6092–6104. [Google Scholar] [CrossRef] [PubMed]
- Nam, R.K.; Amemiya, Y.; Benatar, T.; Wallis, C.J.; Stojcic-Bendavid, J.; Bacopulos, S.; Sherman, C.; Sugar, L.; Naeim, M.; Yang, W.; et al. Identification and validation of a five microRNA signature predictive of prostate cancer recurrence and metastasis: A cohort study. J. Cancer 2015, 6, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Westermann, A.M.; Schmidt, D.; Holdenrieder, S.; Moritz, R.; Semjonow, A.; Schmidt, M.; Kristiansen, G.; Muller, S.C.; Ellinger, J. Serum microRNAs as biomarkers in patients undergoing prostate biopsy: Results from a prospective multi-center study. Anticancer Res. 2014, 34, 665–669. [Google Scholar] [PubMed]
- Shen, J.; Hruby, G.W.; McKiernan, J.M.; Gurvich, I.; Lipsky, M.J.; Benson, M.C.; Santella, R.M. Dysregulation of circulating microRNAs and prediction of aggressive prostate cancer. Prostate 2012, 72, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guan, H.; Wang, Y.; Chen, M.; Xu, B.; Zhang, L.; Lu, K.; Tao, T.; Zhang, X.; Huang, Y. miR-195 inhibits emt by targeting FGF2 in prostate cancer cells. PLoS ONE 2015, 10, e0144073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Qin, X.J.; Cao, D.L.; Zhu, Y.; Yao, X.D.; Zhang, S.L.; Dai, B.; Ye, D.W. An elevated serum miR-141 level in patients with bone-metastatic prostate cancer is correlated with more bone lesions. Asian J. Androl. 2013, 15, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tao, W.; Ni, S.; Chen, Q.; Zhao, Z.; Ma, L.; Fu, Y.; Jiao, Z. Tumor-suppressive microRNA-145 induces growth arrest by targeting SENP1 in human prostate cancer cells. Cancer Sci. 2015, 106, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Mierswa, I.; Wurst, M.; Klinkenberg, R.; Scholz, M.; Euler, T. Yale: Rapid prototyping for complex data mining tasks. In Proceedings of the 12th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Philadelphia, PA, USA, 20–23 August 2006; pp. 935–940.
- Colaprico, A.; Silva, T.C.; Olsen, C.; Garofano, L.; Cava, C.; Garolini, D.; Sabedot, T.S.; Malta, T.M.; Pagnotta, S.M.; Castiglioni, I.; et al. TCGabiolinks: An R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, L.; Batte, K.E.; Trgovcich, J.; Wisler, J.; Marsh, C.B.; Piper, M. Methodological challenges in utilizing miRNAs as circulating biomarkers. J. Cell. Mol. Med. 2014, 18, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.; Fazli, L.; McKenney, J.K.; Simko, J.; Troyer, D.; Nicolas, M.; Newcomb, L.F.; Cowan, J.E.; Crouch, L.; Ferrari, M.; et al. A model for the design and construction of a resource for the validation of prognostic prostate cancer biomarkers: The canary prostate cancer tissue microarray. Adv. Anat. Pathol. 2013, 20, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Huang, X.; Li, Z.; Liu, J.; Wu, J.; Chen, D.; Zhao, F.; Mu, D. miR-199a-3p inhibits aurora kinase a and attenuates prostate cancer growth: New avenue for prostate cancer treatment. Am. J. Pathol. 2014, 184, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Bonci, D.; Coppola, V.; Patrizii, M.; Addario, A.; Cannistraci, A.; Francescangeli, F.; Pecci, R.; Muto, G.; Collura, D.; Bedini, R.; et al. A microRNA code for prostate cancer metastasis. Oncogene 2016, 35, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, F.; Patrawala, L.; Osaki, M.; Takahashi, R.U.; Yamamoto, Y.; Kosaka, N.; Kawamata, M.; Kelnar, K.; Bader, A.G.; Brown, D.; et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol. Ther. 2010, 18, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Zhang, T.; He, D.; Hsieh, J.T. MicroRNA-145 modulates tumor sensitivity to radiation in prostate cancer. Radiat. Res. 2015, 184, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.W.; Lin, J.S.; He, X.X. The emerging role of miR-375 in cancer. Int. J. Cancer. 2014, 135, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yan, W.; He, X.; Zhang, L.; Li, C.; Huang, H.; Nace, G.; Geller, D.A.; Lin, J.; Tsung, A. miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology 2012, 143, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Xu, Y.; Zhang, W.; Deng, Y.; Si, M.; Du, Y.; Yao, H.; Liu, X.; Ke, Y.; Si, J.; et al. miR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010, 20, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.L.; Kwong, D.L.; Chan, T.H.; Law, S.Y.; Chen, L.; Li, Y.; Qin, Y.R.; Guan, X.Y. MicroRNA-375 inhibits tumour growth and metastasis in oesophageal squamous cell carcinoma through repressing insulin-like growth factor 1 receptor. Gut 2012, 61, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Meng, P.; Wang, T.; Qin, W.; Qin, W.; Wang, F.; Yuan, J.; Chen, Z.; Yang, A.; Wang, H. MicroRNA let-7a inhibits proliferation of human prostate cancer cells in vitro and in vivo by targeting E2f2 and CCND2. PLoS ONE 2010, 5, e10147. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.V.; Veliceasa, D.; Vinokour, E.; Volpert, O.V. miR-200b inhibits prostate cancer EMT, growth and metastasis. PLoS ONE 2013, 8, e83991. [Google Scholar] [CrossRef] [PubMed]
- Mercatelli, N.; Coppola, V.; Bonci, D.; Miele, F.; Costantini, A.; Guadagnoli, M.; Bonanno, E.; Muto, G.; Frajese, G.V.; De Maria, R.; et al. The inhibition of the highly expressed miR-221 and miR-222 impairs the growth of prostate carcinoma xenografts in mice. PLoS ONE 2008, 3, e4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bader, A.G. miR-34—A microRNA replacement therapy is headed to the clinic. Front. Genet. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kelnar, K.; Liu, B.; Chen, X.; Calhoun-Davis, T.; Li, H.; Patrawala, L.; Yan, H.; Jeter, C.; Honorio, S.; et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011, 17, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Kojima, K.; Hamada, N.; Ohhashi, R.; Akao, Y.; Nozawa, Y.; Deguchi, T.; Ito, M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem. Biophys. Res. Commun. 2008, 377, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Fujita, Y.; Nozawa, Y.; Deguchi, T.; Ito, M. miR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate 2010, 70, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.A.; Andahur, E.I.; Valenzuela, R.; Castellon, E.A.; Fulla, J.A.; Ramos, C.G.; Trivino, J.C. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 2016, 26, 3993–4008. [Google Scholar]
- Misso, G.; di Martino, M.T.; de Rosa, G.; Farooqi, A.A.; Lombardi, A.; Campani, V.; Zarone, M.R.; Gulla, A.; Tagliaferri, P.; Tassone, P.; et al. miR-34: A new weapon against cancer? Mol. Ther. Nucleic Acids 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Mirna Therapeutics, Inc. microRNA Replacement Therapy. Available online: http://www.mirnarx.com/pipeline/mirna-MRX34.html (accessed on 30 October 2015).




| Intracellular miRNAs | Cohort and Tissue Sample | Discovery Method | Reference |
|---|---|---|---|
| let-7a, miR-20, miR-21, miR-99a, miR-141, miR-182, miR-198; miR-145, miR-155 | 5 PC vs. 4 N cell line | miRNA array and RT-PCR | [11,26] |
| let-7i, miR-25, miR-26a, miR-31, miR-32, miR-34b, miR-92, miR-93, miR-99b, miR-106b, miR-125a, miR-181a, miR-182, miR-188, miR-194, miR-196a, miR-200c, miR-370, miR-375, miR-425, miR-449; let-7b, miR-1, miR-7, miR-34a, miR-126, miR-128a, miR-133a, miR-145, miR-205, miR-218, miR-220, miR-221, miR-329, miR-340, miR-345, miR-410, miR-487, miR-490, miR-494, miR-499, miR-520h | 60 PC vs. 16 N tissues | miRNA array and RT-PCR | [16] |
| miR-148a, miR-200c, miR-375, let-7a, let-7c, let-7f, miR-15b, miR-20a, miR-21, miR-25, miR-26b, miR-30b, miR-106a/b, miR-126-3p, miR-218; miR-143, miR-145; miR-223, miR-22, miR-24, miR-27a, miR-27b, miR-29a, miR-30e, miR-101, miR-125a-5p, miR-125b, miR-152, miR-199a-5p, miR-221, miR-320, miR-424 | 10 PC vs. 10 N tissues | Small RNA cloning and deep sequencing | [28] |
| miR-96, miR-182, miR-375, miR-130b, miR-182-3p, miR-183, miR-524, miR-634; miR-149, miR-181b, miR-205, miR-31, miR-125b, miR-145, miR-184, miR-205, miR-221, miR-222, miR-373, miR-368 | 24 PC vs. 24 N tissues | miRNA array and RT-PCR | [29] |
| miR-200b/c, miR-375, let-7a, miR-17, miR-20a/b, miR-21, miR-93, miR-101, miR-106a/b, miR-141, miR-182, miR-720, miR-768-3p, miR-1274b, miR-1826; miR-145, miR-136-3p, miR-214, miR-221, miR-222,miR-302d-3p, miR-378-3p | 20 PC vs. 20 N tissues | miRNA array and RT-PCR | [30] |
| miR-17, miR-20a/b, miR-93, miR-101, miR-106a, miR-141, miR-145, miR-182, miR-214, miR-221, miR-222, miR-320a, miR-375, miR-720, miR-768-3p | 20 PC vs. 20 N tissues | miRNA array analysis | [31] |
| miR-141, miR-145 and miR-155; let-7a | 75 PC vs. 27 benign samples, blood and tissues | RT-PCR | [32] |
| miR-34*, miR-34c, miR-221, miR-224, miR-182; miR-187 | 50 PC vs. 50 N tissues | miRNA array | [33] |
| Extracellular miRNAs | |||
| miR-7, miR-10a-3p, miR-146b-p, miR-155, miR-181a-2-3p, miR-326, miR-484, miR-485-3p, miR-489, miR-551a, miR-585, miR-601, miR-620, miR-622, miR-659, miR-676-3p, miR-874, miR-943, miR-1252, miR-1253, miR-1273e, miR-1298, miR-1470, miR-1915, miR-3620, miR-3663-5p, miR-3690, miR-4292, miR-4305 | PC3 cell lines and derived exosomes | miRNA array and RT-PCR | [36] |
| miR-141 | xenografted mouse model; sera | RT-PCR | [37] |
| miR-141, miR-298, miR-346, miR-375 | PC adenocarcinoma mouse model; sera | miRNA array and RT-PCR | [38] |
| miR-16, miR-92a/b, miR-103, miR-107,miR-197, miR-34b, miR-328, miR-485-3p, miR-486-5p, miR-574-3p, miR-636, miR-640, miR-766, miR-885-5p | 6 PC vs. 8 N; sera | miRNA array | [39] |
| miR-20b, miR-24, miR-26b, miR-30c, miR-93, miR-106a, miR-223, miR-874, miR-1207-5p, miR-1274a | 29 PC vs. 9 N sera | Multiplex RT-PCR | [40] |
| miR-21, miR-30a, miR-99a, miR-141, miR-200b/c, miR-221, miR-298, miR-346, miR-375 | 25 PC vs. 25 N; sera and SF | RT-PCR | [27,38,41] |
| miR-375, miR-141 | 10 PC metastatic vs. 59 localized PC vs. 48 high risk vs. 23 intermediate risk tumour; sera | miRNA array | [42] |
| miR-93, miR-106a, miR-874, miR-1207-5p, miR-1274a; miR-24, miR-26b, miR-30c, miR-223 | 36 PC vs. 12 N; sera | RT-PCR | [41] |
| let--7i, miR-26a, miR-32, miR-195 | 8 PC vs. 18 BPH vs. 20 N sera | RT-PCR | [43] |
| miR-107, miR-141, miR-375, miR-574-3p | 78 PC vs. 28 N of sera, plasma, urine | RT–PCR microarray | [44] |
| miR-141, miR-210, miR-375, miR-501-3p, miR-551b, miR-562 | 31 PC vs. 13 BPH sera | RT–PCR | [45] |
| miR-346, miR-622, miR-940, miR-1285; let-7e, let-7c, miR-25, miR-30c | 105 PC vs. 61BPH vs. 54 N plasma | miRNA array | [46] |
| miR-15b, miR-200b, miR-218 | 40 localised PC vs. N; whole blood | miRNA array | [47] |
| miR-16, miR-21, miR-34a, miR-125b, miR-141, miR-143, miR-145, miR-155, miR-221, miR-375, miR-425; let-7a | 102 from high Gleason score PC vs. N; whole blood | RT-PCR | [32] |
| miR-15b, let-7c, miR-30c, miR-141, miR-375 | 11 PC vs. 16 BPH; whole blood | RT-PCR | [48] |
| let-7b/c/d/e, miR-17, miR-20a/b, miR-31, miR-100, miR-106a, miR-148a, miR-149, miR-184, miR-196b, miR-200b, miR-429, miR-574-3p, miR-671-3p, miR-150, miR-328 | Urine | RT-PCR | [49] |
| Intracellular miRNAs | Cohort and Tissue Samples | Discovery Method | Reference |
|---|---|---|---|
| miR-31, miR-96, miR-125b, miR-205, miR-222 | 24 PC vs. 24 N; tissue | miRNA array | [29] |
| let-7c, miR-100, miR-145, miR-146, miR-199a and miR-218 | 18 PC vs. 6 BPH; tissue | RT-PCR | [60] |
| miR-1, miR-143, miR-145, miR-205, miR-210, miR-222, miR-451 | 4 PC vs. 4 N; tissue | Deep sequencing | [61] |
| miR-31-5p, miR-34c-5p, miR-96-5p, miR-182-5p, miR-183-5p, miR-205-5p, miR-221-3p and miR-222-3p | Gleason pattern 2 (n = 22), 4 (n = 35) 5 (n = 12); tissue | RT-PCR | [62] |
| miR-29, miR-34a, miR-141 | High Gleason score PC (n = 45); tissue | miRNA array | [63] |
| let-7a/c, miR-146b, miR-181b, miR-361, miR-515-3p/5p | Different PC risk groups (n = 98); tissue | miRNA array | [64] |
| miR-182, miR-187 | Gleason ≤ 7 (n = 302) or >7 (n = 46) | miRNA array | [33] |
| miR-96-5p, miR-145-5p, miR-183-5p, miR-221-5p; definition of miQ index | n = 25 PC vs. n = 25 N tissues | RT-PCR | [65] |
| miR-182, miR-375 | n = 119 PC patients | miRNA array | [67] |
| miR-301a, miR-652, miR-454, miR-223 and miR-139 | n = 515 PC patients (metastatic vs. non-metastatic) | NGS | [74] |
| Single prognostic miRNA | |||
| miR-182 | n = 147 PC patients | RT-PCR | [68] |
| miR-30c, miR-203 | n = 44 PC patients | RT-PCR | [69] |
| miR-101 | n = 16 localized PC, n = 26 metastatic PC | RT-PCR | [70] |
| miR-195 | n = 225 PC patients | RT-PCR | [71] |
| miR-150 | n = 167 PC patients | RT-PCR | [72] |
| miR-188-5p | n = 180 PC patients | RT-PCR | [73] |
| Extracellular miRNAs | |||
| miR-24, miR-93, miR-106a, miR-451 | different risk PC (n = 29) (CAPRA score); sera | Multiplex RT-PCR | [40] |
| miR-141, miR-200b and miR-375 | 14 primary or 7 metastatic PC men; sera | miRNA array | [42] |
| miR-195 and let-7i | 37 localized PC, 18 BPH, 8 metastatic PC and 20 N; sera | RT-PCR | [43] |
| miR-141 is increased in higher Gleason score patients | from 133 patients; sera | RT–PCR | [75] |
| miR-21 and miR-145 in patients with intermediate- or high-risk miR-20a and miR-21 in high-risk miR-20a, miR-21, miR-145 and miR-221 in high- vs. low-risk PC men | low-, intermediate- and high-risk patients (82 patients); plasma | RT–PCR | [76] |
| miR-141, miR-200b and miR-375 | 55 localized PC patients vs. 11 metastatic PC; plasma | RT–PCR microarray | [44] |
| let-7a, miR-141, miR-145 and miR-155 | 75 PC and 27 N Blood and tissues | RT-PCR | [32] |
| miR-1246, miR-1290, miR-375 | 100 late-stage PC patients; blood | RT-PCR | [69] |
| Classification | 29 miRNA Diagnostic Signature | Accuracy | AUC |
|---|---|---|---|
| PC vs. N 52 vs. 52 | Let-7a/b/c/i, miR-15b, miR-17, miR-20a, miR-21, miR-24, miR-25, miR-26a/b, miR-31, miR-32, miR-34b, miR-93, miR-106a, miR-141, miR-143, miR-145, miR-148a, miR-155, miR-182, miR-187, miR-200b, miR-218, miR-221, miR-223, miR-375 | 97.18% +/− 4.31% (CI 95%): 96.35–98.00 | 0.989 +/− 0.016 (CI 95%): 98.59–99.20 |
| Classification | 7 miRNA Prognostic Signature | Accuracy | AUC |
|---|---|---|---|
| P1 + P2 vs. P5 138 vs. 138 | let-7a, miR-141, miR-145, miR-195, miR-221, miR-375, miR-451 | 71.38% (CI 95%): 70.79–71.96 | 74.7% (CI 95%): 73.28–76.11 |
| P3 + P4 vs. P5 138 vs. 138 | let-7a, miR-141, miR-145, miR-195, miR-221, miR-375, miR-451 | 61.59% (CI 95%): 60.71–62.46 | 61.6% (CI 95%): 60.24–62.95 |
| P1 + P2 vs. P3 + P4 164 vs. 164 | let-7a, miR-141, miR-145, miR-195, miR-221, miR-375, miR-451 | 65.26% (CI 95%): 64.51–66.00 | 66.8% (CI 95%): 66.10–67.49 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoli, G.; Cava, C.; Castiglioni, I. MicroRNAs as Biomarkers for Diagnosis, Prognosis and Theranostics in Prostate Cancer. Int. J. Mol. Sci. 2016, 17, 421. https://doi.org/10.3390/ijms17030421
Bertoli G, Cava C, Castiglioni I. MicroRNAs as Biomarkers for Diagnosis, Prognosis and Theranostics in Prostate Cancer. International Journal of Molecular Sciences. 2016; 17(3):421. https://doi.org/10.3390/ijms17030421
Chicago/Turabian StyleBertoli, Gloria, Claudia Cava, and Isabella Castiglioni. 2016. "MicroRNAs as Biomarkers for Diagnosis, Prognosis and Theranostics in Prostate Cancer" International Journal of Molecular Sciences 17, no. 3: 421. https://doi.org/10.3390/ijms17030421
APA StyleBertoli, G., Cava, C., & Castiglioni, I. (2016). MicroRNAs as Biomarkers for Diagnosis, Prognosis and Theranostics in Prostate Cancer. International Journal of Molecular Sciences, 17(3), 421. https://doi.org/10.3390/ijms17030421