Fluorescence-Lifetime Imaging Microscopy for Visualization of Quantum Dots’ Endocytic Pathway
Abstract
:1. Introduction
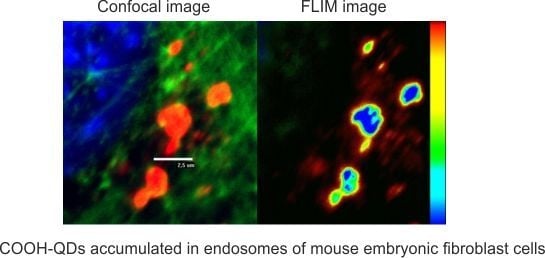
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| QDs | Quantum dots |
| PL | Photoluminescence |
| FLIM | Fluoscence-lifetime imaging microscopy |
| PBS | phosphate buffer saline |
| NIH3T3 | immortalized mouse embryonic fibroblast cells |
| PEG | polyethylene glycol |
| MPA | mercaptopropionic acid |
References
- Becker, W. Fluorescence lifetime imaging—Techniques and applications. J. Microsc. 2012, 247, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Suhling, K.; Hirvonen, L.M.; Levitt, J.A.; Chung, P.H.; Tregidgo, C.; Rusakov, D.A.; Zheng, K.; Ameer-Beg, S.; Coelho, S.; Henderson, R.; et al. Fluorescence Lifetime Imaging Handbook of Photonics for Biomedical Engineering; Springer: New York, NY, USA, 2015; pp. 1–50. [Google Scholar]
- Zhang, L.; Lei, J.; Liu, J.; Ma, F.; Ju, H. Persistent luminescence nanoprobe for biosensing and lifetime imaging of cell apoptosis via time-resolved fluorescence resonance energy transfer. Biomaterials 2015, 67, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Ruck, A.; Hulshoff, C.; Kinzler, I.; Becker, W.; Steiner, R. SLIM: A new method for molecular imaging. Microsc. Res. Tech. 2007, 70, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Horilova, J.; Mateasik, A.; Revilla-i-Domingo, R.; Raible, F.; Chorvat, D., Jr.; Chorvatova, A.M. Fingerprinting of metabolic states by NAD(P)H fluorescence lifetime spectroscopy in living cells: A review. Med. Photonics 2015, 27, 62–69. [Google Scholar] [CrossRef]
- Van Munster, E.B.; Gadella, T.W.J. Fluorescence lifetime imaging microscopy (FLIM). Microsc. Tech. Adv. Biochem. Eng. 2005, 95, 143–175. [Google Scholar]
- Lu, Y.; Zhao, J.; Zhang, R.; Liu, Y.; Liu, D.; Goldys, E.M.; Yang, X.; Xi, P.; Sunna, A.; Lu, J.; et al. Tunable lifetime multiplexing using luminescent nanocrystals. Nat. Photonics 2014, 8, 32–36. [Google Scholar] [CrossRef]
- Ishikawa-Ankerhold, H.C.; Ankerhold, R.; Drummen, G.P.C. Advanced fluorescence microscopy techniques—FRAP, FLIP, FLAP, FRET and FLIM. Molecules 2012, 17, 4047–4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Behnke, T.; Drescher, D.; Kneipp, J.; Resch-Genger, U. Near-infrared-emitting nanoparticles for lifetime-based multiplexed analysis and imaging of living cells. ACS Nano 2013, 7, 6674–6684. [Google Scholar] [CrossRef] [PubMed]
- Basuki, J.B.; Duong, H.T.T.; Macmillan, A.; Erlich, R.B.; Esser, L.; Akerfeldt, M.C.; Whan, R.M.; Kavallaris, M.; Boyer, C.; Davis, T.P. Using Fluorescence lifetime imaging microscopy to monitor theranostic nanoparticle uptake and intracellular doxorubicin release. ACS Nano 2013, 7, 10175–10189. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, J.; Fuji, M.; Sakka, M.; Zhu, Y.; Asahara, T.; Shirahata, N.; Ii, M.; Bai, Z.; Li, J.; et al. Highly fluorescent silica-coated bismuth-doped aluminosilicate nanoparticles for near-infrared bioimaging. Small 2011, 7, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.; Hsu, T.; Wu, Y.; Hsu, J.; Chang, H. Fluorescence lifetime imaging microscopy of nanodiamonds in vivo SPIE 8635. Adv. Photonics Quantum Comput. Mem. Commun. 2013, 863503. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Orte, A.; Alvarez-Pez, J.M.; Ruedas-Rama, M.J. Fluorescence lifetime imaging microscopy for the detection of intracellular pH with quantum dot nanosensors. ACS Nano 2013, 7, 6387–6395. [Google Scholar] [CrossRef] [PubMed]
- Carlini, L.; Nadeau, J.L. Uptake and processing of semiconductor quantum dots in living cells studied by fluorescence lifetime imaging microscopy (FLIM). Chem. Commun. 2013, 49. [Google Scholar] [CrossRef] [PubMed]
- Steponkiene, S.; Valanciunaite, J.; Skripka, A.; Rotomskis, R. Cellular uptake and photosensitizing properties of quantum dot-chlorin e(6) complex: In vitro study. J. Biomed. Nanotechnol. 2013, 10, 679–686. [Google Scholar] [CrossRef]
- Valanciunaite, J.; Klymchenko, A.S.; Skripka, A.; Richert, L.; Steponkiene, S.; Streckyte, G.; Melyc, Y.; Rotomskis, R. Non-covalent complex of quantum dots and chlorin e6: Efficient energy transfer and remarkable stability in living cells. RSC Adv. 2014, 4, 52270–52278. [Google Scholar] [CrossRef]
- Damalakiene, L.; Karabanovas, V.; Bagdonas, S.; Valius, M.; Rotomskis, R. Intracellular distribution of non targeted quantum dots after natural uptake and microinjection. Int. J. Nanomed. 2013, 8, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Karabanovas, V.; Zitkus, Z.; Kuciauskas, D.; Rotomskis, R.; Valius, M. Surface properties of quantum dots define their cellular endocytic routes, mitogenic stimulation and suppression of cell migration. J. Biomed. Nanotechnol. 2014, 10, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, R.; DeRobertis, E.M. Endocytic control of growth factor signalling: Multivesicular bodies as signalling organelles. Nat. Rev. Mol. Cell Biol. 2012, 13, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Piper, R.C.; Luzio, J.P. Late endosomes: sorting and partitioning in multivesicular bodies. Traffic 2001, 2, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Sun, Y.H.; Vernier, P.T.; Liang, C.H.; Chong, S.Y.C.; Gundersen, M.A. pH-sensitive photoluminescence of CdSe/ZnSe/ZnS quantum dots in human ovarian cancer cells. J. Phys. Chem. 2007, 111, 2872–2878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mi, L.; Chen, J.; Wang, P. The environmental influence on the photoluminescence behavior of thiol-capped CdTe quantum dots in living cells. Biomed. Mater. 2009, 4. [Google Scholar] [CrossRef]
- Conroy, J.; Byrne, S.J.; Gunko, Y.K.; Rakovich, Y.P.; Donegan, J.F.; Davies, A.; Kelleher, D.; Volkov, Y. CdTe nanoparticles display tropism to core histones and histone-rich cell organelles. Small 2008, 4, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Kalwarczyk, E.; Ziębacz, N.; Kalwarczyk, T.; Hołyst, R.; Fiałkowski, M. A “wrap-and-wrest” mechanism of fluorescence quenching of CdSe/ZnS quantum dots by surfactant molecules. Nanoscale 2013, 5, 9908–9916. [Google Scholar] [CrossRef] [PubMed]




| pH Value | pH 4.0 | pH 4.5 | pH 5.0 | pH 5.5 | pH 6.0 | pH 7.2 |
|---|---|---|---|---|---|---|
| PL lifetime τ (ns) | 14.7 ± 0.6 | 15.7 ± 0.5 | 15.7 ± 0.5 | 16.5 ± 0.50 | 17.6 ± 0.2 | 19.0 ± 0.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damalakiene, L.; Karabanovas, V.; Bagdonas, S.; Rotomskis, R. Fluorescence-Lifetime Imaging Microscopy for Visualization of Quantum Dots’ Endocytic Pathway. Int. J. Mol. Sci. 2016, 17, 473. https://doi.org/10.3390/ijms17040473
Damalakiene L, Karabanovas V, Bagdonas S, Rotomskis R. Fluorescence-Lifetime Imaging Microscopy for Visualization of Quantum Dots’ Endocytic Pathway. International Journal of Molecular Sciences. 2016; 17(4):473. https://doi.org/10.3390/ijms17040473
Chicago/Turabian StyleDamalakiene, Leona, Vitalijus Karabanovas, Saulius Bagdonas, and Ricardas Rotomskis. 2016. "Fluorescence-Lifetime Imaging Microscopy for Visualization of Quantum Dots’ Endocytic Pathway" International Journal of Molecular Sciences 17, no. 4: 473. https://doi.org/10.3390/ijms17040473
APA StyleDamalakiene, L., Karabanovas, V., Bagdonas, S., & Rotomskis, R. (2016). Fluorescence-Lifetime Imaging Microscopy for Visualization of Quantum Dots’ Endocytic Pathway. International Journal of Molecular Sciences, 17(4), 473. https://doi.org/10.3390/ijms17040473