Matrilin-3 Role in Cartilage Development and Osteoarthritis
Abstract
:1. Introduction
1.1. Articular Cartilage and Osteoarthritis
1.2. Changes in the Remodeling Balance of Cartilage
2. Role of Matrilin-3
2.1. Matrilin-3: Bone-Cartilage ECM Modulator
2.2. Role of Matrilin-3 in Chondrogenesis, Terminal Differentiation of Chondrocytes, and Ossification
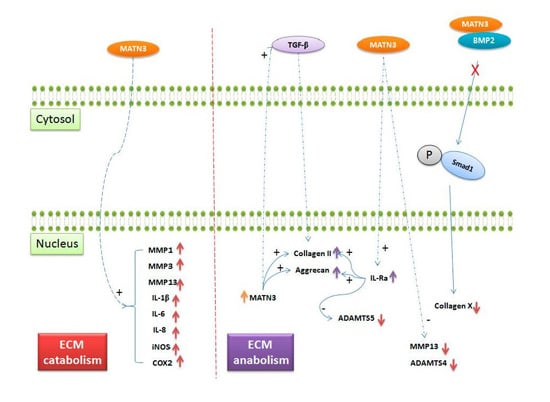
2.3. Role of Matrilin-3 in Osteoarthritis
3. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TGF-β | Transforming growth factor β |
| BMP | Bone morphogenetic protein |
| GDF5 | Growth differentiation factor 5 |
| FRZB | Secreted frizzled-related protein 3 |
| MATN3 | Matrilin-3 |
| ECM | Extracellular matrix |
| IL | Interleukin-1 |
| MMPs | Matrix metalloproteinases |
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| VEGF | Vascular endothelial growth factor |
| RUNX2 | Runt-related transcription factor |
| VWFA | Von Willebrand factor A |
| EGF | Epidermal growth factor |
| MED | Multiple epiphyseal dysplasia |
| SEMD | Spondyloepimetaphyseal dysplasia |
References
- Temenoff, J.S.; Mikos, A.G. Review: Tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Felson, D.T. Osteoarthritis. BMJ 2006, 332, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Soltz, M.A.; Ateshian, G.A. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann. Biomed. Eng. 2000, 28, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.P. Articular cartilage repair. Am. J. Sports Med. 1998, 26, 309–324. [Google Scholar] [PubMed]
- Madry, H.; van Dijk, C.N.; Mueller-Gerbl, M. The basic science of the subchondral bone. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2010, 18, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat. Biotechnol. 1998, 16, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T. Clinical practice. Osteoarthritis of the knee. N. Engl. J. Med. 2006, 354, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E. Clinical practice. Osteoarthritis of the hip. N. Engl. J. Med. 2007, 357, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.M.; Bathon, J.M. Osteoarthritis in older adults. J. Am. Geriatr. Soc. 1998, 46, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Anderson, J.J.; Meenan, R.F. The comparative efficacy and toxicity of second-line drugs in rheumatoid arthritis. Results of two metaanalyses. Arthritis Rheum. 1990, 33, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, L.; Olsen, B.R. Lessons from genetic forms of osteoarthritis for the pathogenesis of the disease. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2007, 15, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Mabuchi, A.; Shi, D.; Kubo, T.; Takatori, Y.; Saito, S.; Fujioka, M.; Sudo, A.; Uchida, A.; Yamamoto, S.; et al. A functional polymorphism in the 5′UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat. Genet. 2007, 39, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Spector, T.D. The contribution of genes to osteoarthritis. Rheum. Dis. Clin. N. Am. 2008, 34, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Spector, T.D. The clinical relevance of genetic susceptibility to osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2010, 24, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Southam, L.; Rodriguez-Lopez, J.; Wilkins, J.M.; Pombo-Suarez, M.; Snelling, S.; Gomez-Reino, J.J.; Chapman, K.; Gonzalez, A.; Loughlin, J. An SNP in the 5′-UTR of GDF5 is associated with osteoarthritis susceptibility in europeans and with in vivo differences in allelic expression in articular cartilage. Hum. Mol. Genet. 2007, 16, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, E.; Chapman, K.; Meulenbelt, I.; Karassa, F.B.; Loughlin, J.; Carr, A.; Doherty, M.; Doherty, S.; Gomez-Reino, J.J.; Gonzalez, A.; et al. Large-scale analysis of association between GDF5 and FRZB variants and osteoarthritis of the hip, knee, and hand. Arthritis Rheum. 2009, 60, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Rong, J.; Guan, F.; Jiang, L.; Tao, S.; Guan, G.; Tao, T. MATN3 gene polymorphism is associated with osteoarthritis in Chinese Han population: A community-based case-control study. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Pullig, O.; Tagariello, A.; Schweizer, A.; Swoboda, B.; Schaller, P.; Winterpacht, A. MATN3 (matrilin-3) sequence variation (pT303M) is a risk factor for osteoarthritis of the CMC1 joint of the hand, but not for knee osteoarthritis. Ann. Rheum. Dis. 2007, 66, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Krakow, D.; Rimoin, D.L. The skeletal dysplasias. Genet. Med. 2010, 12, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Kwan Tat, S.; Lajeunesse, D.; Pelletier, J.P.; Martel-Pelletier, J. Targeting subchondral bone for treating osteoarthritis: What is the evidence? Best Pract. Res. Clin. Rheumatol. 2010, 24, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Leeming, D.J.; Dam, E.B.; Henriksen, K.; Alexandersen, P.; Pastoureau, P.; Altman, R.D.; Christiansen, C. Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2008, 16, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Hu, J.; Zhou, Q.; Wang, J.; Liu, P.; Wei, Y. Application of co-expressed genes to articular cartilage: New hope for the treatment of osteoarthritis (review). Mol. Med. Rep. 2012, 6, 16–18. [Google Scholar] [PubMed]
- Ansboro, S.; Greiser, U.; Barry, F.; Murphy, M. Strategies for improved targeting of therapeutic cells: Implications for tissue repair. Eur. Cells Mater. 2012, 23, 310–318. [Google Scholar]
- Lories, R.J.; Luyten, F.P. The bone-cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Luyten, F.P.; Lories, R.J.; Verschueren, P.; de Vlam, K.; Westhovens, R. Contemporary concepts of inflammation, damage and repair in rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 2006, 20, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Thambyah, A.; Broom, N. On new bone formation in the pre-osteoarthritic joint. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2009, 17, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.L.; Thaeler-Oberdoerster, D.A.; Greenwald, A.S. Subchondral pathways to the superior surface of the human talus. Foot Ankle 1986, 7, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Imhof, H.; Breitenseher, M.; Kainberger, F.; Rand, T.; Trattnig, S. Importance of subchondral bone to articular cartilage in health and disease. Top. Magn. Reson. Imaging TMRI 1999, 10, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.J.; McClure, S.F.; Stoddart, R.W.; McClure, J. The normal human chondro-osseous junctional region: Evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Musculoskelet. Disord. 2006, 7. [Google Scholar] [CrossRef]
- Burr, D.B. The importance of subchondral bone in osteoarthrosis. Curr. Opin. Rheumatol. 1998, 10, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J.; Mansell, J.P. Do subchondral bone changes exacerbate or precede articular cartilage destruction in osteoarthritis of the elderly? Gerontology 1997, 43, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Wagener, R.; Ehlen, H.W.; Ko, Y.P.; Kobbe, B.; Mann, H.H.; Sengle, G.; Paulsson, M. The matrilins—Adaptor proteins in the extracellular matrix. FEBS Lett. 2005, 579, 3323–3329. [Google Scholar] [CrossRef] [PubMed]
- Klatt, A.R.; Becker, A.K.; Neacsu, C.D.; Paulsson, M.; Wagener, R. The matrilins: Modulators of extracellular matrix assembly. Int. J. Biochem. Cell Biol. 2011, 43, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Wagener, R.; Kobbe, B.; Paulsson, M. Primary structure of matrilin-3, a new member of a family of extracellular matrix proteins related to cartilage matrix protein (matrilin-1) and Von Willebrand factor. FEBS Lett. 1997, 413, 129–134. [Google Scholar] [CrossRef]
- Deak, F.; Wagener, R.; Kiss, I.; Paulsson, M. The matrilins: A novel family of oligomeric extracellular matrix proteins. Matrix Biol. J. Int. Soc. Matrix Biol. 1999, 18, 55–64. [Google Scholar] [CrossRef]
- Klatt, A.R.; Nitsche, D.P.; Kobbe, B.; Morgelin, M.; Paulsson, M.; Wagener, R. Molecular structure and tissue distribution of matrilin-3, a filament-forming extracellular matrix protein expressed during skeletal development. J. Biol. Chem. 2000, 275, 3999–4006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Q. Changes of matrilin forms during endochondral ossification. Molecular basis of oligomeric assembly. J. Biol. Chem. 2000, 275, 32628–32634. [Google Scholar] [CrossRef] [PubMed]
- Fresquet, M.; Jowitt, T.A.; Ylostalo, J.; Coffey, P.; Meadows, R.S.; Ala-Kokko, L.; Thornton, D.J.; Briggs, M.D. Structural and functional characterization of recombinant matrilin-3 A-domain and implications for human genetic bone diseases. J. Biol. Chem. 2007, 282, 34634–34643. [Google Scholar] [CrossRef] [PubMed]
- Mostert, A.K.; Dijkstra, P.F.; Jansen, B.R.; van Horn, J.R.; de Graaf, B.; Heutink, P.; Lindhout, D. Familial multiple epiphyseal dysplasia due to a matrilin-3 mutation: Further delineation of the phenotype including 40 years follow-up. Am. J. Med. Genet. Part A 2003, 120A, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Borochowitz, Z.U.; Scheffer, D.; Adir, V.; Dagoneau, N.; Munnich, A.; Cormier-Daire, V. Spondylo-epi-metaphyseal dysplasia (SEMD) matrilin 3 type: Homozygote matrilin 3 mutation in a novel form of semd. J. Med. Genet. 2004, 41, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, A.; Haga, N.; Maeda, K.; Nakashima, E.; Manabe, N.; Hiraoka, H.; Kitoh, H.; Kosaki, R.; Nishimura, G.; Ohashi, H.; et al. Novel and recurrent mutations clustered in the Von Willebrand factor a domain of MATN3 in multiple epiphyseal dysplasia. Hum. Mutat. 2004, 24, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, C.T.; Goldring, M.B.; Terek, R.; Chen, Q. Matrilin-3 induction of Il-1 receptor antagonist is required for up-regulating collagen II and aggrecan and down-regulating ADAMTs-5 gene expression. Arthritis Res. Ther. 2012, 14. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Luo, J.; Chen, Q. Enhancing and maintaining chondrogenesis of synovial fibroblasts by cartilage extracellular matrix protein matrilins. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2008, 16, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Van der Weyden, L.; Wei, L.; Luo, J.; Yang, X.; Birk, D.E.; Adams, D.J.; Bradley, A.; Chen, Q. Functional knockout of the matrilin-3 gene causes premature chondrocyte maturation to hypertrophy and increases bone mineral density and osteoarthritis. Am. J. Pathol. 2006, 169, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.L.; Mortier, G.R.; Chapman, K.; Loughlin, J.; Grant, M.E.; Briggs, M.D. Mutations in the region encoding the Von Willebrand factor a domain of matrilin-3 are associated with multiple epiphyseal dysplasia. Nat. Genet. 2001, 28, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Otten, C.; Wagener, R.; Paulsson, M.; Zaucke, F. Matrilin-3 mutations that cause chondrodysplasias interfere with protein trafficking while a mutation associated with hand osteoarthritis does not. J. Med. Genet. 2005, 42, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Liu, J.S.; Chen, J.Y.; Wu, S.Q.; Wang, G.R.; Nie, J.; Zhang, S.K.; Guo, Q.L.; Luo, J.M. Multiple functions of the first EGF domain in matrilin-3: Secretion and endoplasmic reticulum stress. Int. J. Mol. Med. 2015, 36, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, S.L.; Jackson, G.C.; Leighton, M.P.; Wagener, R.; Makitie, O.; Cole, W.G.; Briggs, M.D. Multiple epiphyseal dysplasia mutations in MATN3 cause misfolding of the A-domain and prevent secretion of mutant matrilin-3. Hum. Mutat. 2005, 26, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, C.T.; Zhou, F.H.; Pei, M.; Wang, Z.; Lemme, N.J.; Haines, P.; Chen, Q. Matrilin-3 chondrodysplasia mutations cause attenuated chondrogenesis, premature hypertrophy and aberrant response to TGF-β in chondroprogenitor cells. Int. J. Mol. Sci. 2014, 15, 14555–14573. [Google Scholar] [CrossRef] [PubMed]
- Volk, S.W.; Luvalle, P.; Leask, T.; Leboy, P.S. A BMP responsive transcriptional region in the chicken type X collagen gene. J. Bone Miner. Res. 1998, 13, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Trehan, S.K.; Guan, Y.; Sun, C.; Moore, D.C.; Jayasuriya, C.T.; Chen, Q. Matrilin-3 inhibits chondrocyte hypertrophy as a bone morphogenetic protein-2 antagonist. J. Biol. Chem. 2014, 289, 34768–34779. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, S.E.; Jonsson, H.; Ingvarsson, T.; Manolescu, I.; Jonsson, H.H.; Olafsdottir, G.; Palsdottir, E.; Stefansdottir, G.; Sveinbjornsdottir, G.; Frigge, M.L.; et al. Genomewide scan for hand osteoarthritis: A novel mutation in matrilin-3. Am. J. Hum. Genet. 2003, 72, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Pullig, O.; Weseloh, G.; Klatt, A.R.; Wagener, R.; Swoboda, B. Matrilin-3 in human articular cartilage: Increased expression in osteoarthritis. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2002, 10, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Mann, H.H.; Sengle, G.; Gebauer, J.M.; Eble, J.A.; Paulsson, M.; Wagener, R. Matrilins mediate weak cell attachment without promoting focal adhesion formation. Matrix Biol. J. Int. Soc. Matrix Biol. 2007, 26, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Klatt, A.R.; Klinger, G.; Paul-Klausch, B.; Kuhn, G.; Renno, J.H.; Wagener, R.; Paulsson, M.; Schmidt, J.; Malchau, G.; Wielckens, K. Matrilin-3 activates the expression of osteoarthritis-associated genes in primary human chondrocytes. FEBS Lett. 2009, 583, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Klatt, A.R.; Paul-Klausch, B.; Klinger, G.; Hillebrand, U.; Kuhn, G.; Kobbe, B.; Renno, J.H.; Johannis, W.; Paulsson, M.; Wagener, R. The matrilin-3 VWA1 domain modulates interleukin-6 release from primary human chondrocytes. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2013, 21, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Vincourt, J.B.; Etienne, S.; Grossin, L.; Cottet, J.; Bantsimba-Malanda, C.; Netter, P.; Mainard, D.; Libante, V.; Gillet, P.; Magdalou, J. Matrilin-3 switches from anti- to pro-anabolic upon integration to the extracellular matrix. Matrix Biol. J. Int. Soc. Matrix Biol. 2012, 31, 290–298. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muttigi, M.S.; Han, I.; Park, H.-K.; Park, H.; Lee, S.-H. Matrilin-3 Role in Cartilage Development and Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 590. https://doi.org/10.3390/ijms17040590
Muttigi MS, Han I, Park H-K, Park H, Lee S-H. Matrilin-3 Role in Cartilage Development and Osteoarthritis. International Journal of Molecular Sciences. 2016; 17(4):590. https://doi.org/10.3390/ijms17040590
Chicago/Turabian StyleMuttigi, Manjunatha S., Inbo Han, Hun-Kuk Park, Hansoo Park, and Soo-Hong Lee. 2016. "Matrilin-3 Role in Cartilage Development and Osteoarthritis" International Journal of Molecular Sciences 17, no. 4: 590. https://doi.org/10.3390/ijms17040590
APA StyleMuttigi, M. S., Han, I., Park, H.-K., Park, H., & Lee, S.-H. (2016). Matrilin-3 Role in Cartilage Development and Osteoarthritis. International Journal of Molecular Sciences, 17(4), 590. https://doi.org/10.3390/ijms17040590