Prototype and Chimera-Type Galectins in Placentas with Spontaneous and Recurrent Miscarriages
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Galectin Expression in First-Trimester Placenta with Spontaneous and Recurrent Abortion
2.2.1. Galectin-1 Expression in the Villous Trophoblast Is Significantly Lowered in Comparison to Healthy First Pregnancy Placentas
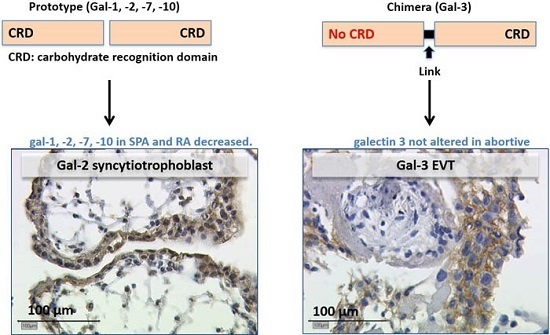
2.2.2. In SPA and RA Placentas Expression of Galectin-2 Is Decreased in both Compartments
2.2.3. Expression of Galectin-3 Is Not Altered in Abortive Placentas
2.2.4. Galectin-7 Expression Is Lowered in Villous Trophoblasts
2.2.5. In SPA and RA, Galectin-10 Is Lowered in the Villous Trophoblast
2.3. Correlation Analysis
3. Discussion
3.1. Decreased Gal-1 Expression in the Trophoblast Is Correlated with Miscarriage
3.2. Gal-2 Is Expressed on Decreased Levels in Placentas after Spontaneous and Recurrent Abortion
3.3. Gal-3 as Chimera-Type Galectin Shows No Significant Difference of Expression in Spontaneous and Recurrent Abortions
3.4. Gal-7 Expression Is Lowered in SPA and RA Placentas
3.5. The Staining of Galectin-10 Is Strong, but Decreasing in SPA and RA Placentas
4. Materials and Methods
4.1. Tissue Samples
4.2. Immunohistochemistry
4.3. Double Immunofluorescence Staining
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rubio, C.; Simon, C.; Vidal, F.; Rodrigo, L.; Pehlivan, T.; Remohi, J.; Pellicer, A. Chromosomal abnormalities and embryo development in recurrent miscarriage couples. Hum. Reprod. 2003, 18, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Rani, R.; Agrawal, S. An update in recurrent spontaneous abortion. Arch. Gynecol. Obstet. 2005, 272, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Bro, S.P.; Kjaersgaard, M.I.; Parner, E.T.; Sorensen, M.J.; Olsen, J.; Bech, B.H.; Pedersen, L.H.; Christensen, J.; Vestergaard, M. Adverse pregnancy outcomes after exposure to methylphenidate or atomoxetine during pregnancy. Clin. Epidemiol. 2015, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, I.P.; Foarfa, M.C.; Pirlog, M.C.; Turculeanu, A. Prenatal depression and stress—Risk factors for placental pathology and spontaneous abortion. Romanian J. Morphol. Embryol. 2014, 55, 1155–1160. [Google Scholar]
- Genc, M.; Genc, B.; Cengiz, H. Adenomyosis and accompanying gynecological pathologies. Arch. Gynecol. Obstet. 2015, 291, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Jaslow, C.R. Uterine factors. Obstet. Gynecol. Clin. N. Am. 2014, 41, 57–86. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Haufe, T.; Scholz, C.; Kuhn, C.; Friese, K.; Karamouti, M.; Makrigiannakis, A.; Jeschke, U. Placental interleukin-15 expression in recurrent miscarriage. Am. J. Reprod. Immunol. 2010, 64, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Mosmann, T.R.; Guilbert, L.; Tuntipopipat, S.; Wegmann, T.G. Synthesis of t helper 2-type cytokines at the maternal-fetal interface. J. Immunol. (Baltimore, Md. : 1950) 1993, 151, 4562–4573. [Google Scholar]
- Ziegelmuller, B.; Vattai, A.; Kost, B.; Kuhn, C.; Hofmann, S.; Bayer, B.; Toth, B.; Jeschke, U.; Ditsch, N. Expression of thyroid hormone receptors in villous trophoblasts and decidual tissue at protein and mrna levels is downregulated in spontaneous and recurrent miscarriages. J. Histochem. Cytochem. 2015, 63, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar] [PubMed]
- Brewer, C.F. Lectin cross-linking interactions with multivalent carbohydrates. Adv. Exp. Med. Biol. 2001, 491, 17–25. [Google Scholar] [PubMed]
- Jeschke, U.; Hutter, S.; Heublein, S.; Vrekoussis, T.; Andergassen, U.; Unverdorben, L.; Papadakis, G.; Makrigiannakis, A. Expression and function of galectins in the endometrium and at the human feto-maternal interface. Placenta 2013, 34, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Havel, P.J.; Liu, F.T. Galectin-12: A protein associated with lipid droplets that regulates lipid metabolism and energy balance. Adipocyte 2012, 1, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Paclik, D.; Werner, L.; Guckelberger, O.; Wiedenmann, B.; Sturm, A. Galectins distinctively regulate central monocyte and macrophage function. Cell. Immunol. 2011, 271, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Romero, R.; Goodman, M.; Weckle, A.; Xing, J.; Dong, Z.; Xu, Y.; Tarquini, F.; Szilagyi, A.; Gal, P.; et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc. Natl. Acad. Sci. USA 2009, 106, 9731–9736. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.M.; Ilarregui, J.M.; Tometten, M.; Garcia, M.; Orsal, A.S.; Cordo-Russo, R.; Toscano, M.A.; Bianco, G.A.; Kobelt, P.; Handjiski, B.; et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat. Med. 2007, 13, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Cedeno-Laurent, F.; Dimitroff, C.J. Galectin-1 research in T cell immunity: Past, present and future. Clin. Immunol. 2012, 142, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Hutter, S.; Knabl, J.; Andergassen, U.; Mayr, D.; Hofmann, S.; Kuhn, C.; Mahner, S.; Arck, P.; Jeschke, U. Fetal gender specific expression of tandem-repeat galectins in placental tissue from normally progressed human pregnancies and intrauterine growth restriction (IUGR). Placenta 2015, 36, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.M.; Barrientos, G. Galectin signature in normal pregnancy and preeclampsia. J. Reprod. Immunol. 2013, 101–102, 127–134. [Google Scholar]
- Than, N.G.; Erez, O.; Wildman, D.E.; Tarca, A.L.; Edwin, S.S.; Abbas, A.; Hotra, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.S.; et al. Severe preeclampsia is characterized by increased placental expression of galectin-1. J. Matern.-Fetal Neonatal Med. 2008, 21, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Hutter, S.; Martin, N.; von Schonfeldt, V.; Messner, J.; Kuhn, C.; Hofmann, S.; Andergassen, U.; Knabl, J.; Jeschke, U. Galectin 2 (gal-2) expression is downregulated on protein and mrna level in placentas of preeclamptic (pe) patients. Placenta 2015, 36, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Unverdorben, L.; Huttenbrenner, R.; Knabl, J.; Jeschke, U.; Hutter, S. Galectin-13/pp-13 expression in term placentas of gestational diabetes mellitus pregnancies. Placenta 2015, 36, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta 2002, 1572, 232–254. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Kasai, K. The family of metazoan metal-independent beta-galactoside-binding lectins: Structure, function and molecular evolution. Glycobiology 1993, 3, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R. Galectins as pattern recognition receptors: Structure, function, and evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [PubMed]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.; et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Lopez-Lucendo, M.F.; Solis, D.; Andre, S.; Hirabayashi, J.; Kasai, K.; Kaltner, H.; Gabius, H.J.; Romero, A. Growth-regulatory human galectin-1: Crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J. Mol. Biol. 2004, 343, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Gonzalez, I.; Freitag, N.; Barrientos, G.; Shaikly, V.; Nagaeva, O.; Strand, M.; Kjellberg, L.; Klapp, B.F.; Mincheva-Nilsson, L.; Cohen, M.; et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Hum. Reprod. 2013, 19, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Maquoi, E.; van den Brule, F.A.; Castronovo, V.; Foidart, J.M. Changes in the distribution pattern of galectin-1 and galectin-3 in human placenta correlates with the differentiation pathways of trophoblasts. Placenta 1997, 18, 433–439. [Google Scholar] [CrossRef]
- Jeschke, U.; Mayr, D.; Schiessl, B.; Mylonas, I.; Schulze, S.; Kuhn, C.; Friese, K.; Walzel, H. Expression of galectin-1, -3 (gal-1, gal-3) and the thomsen-friedenreich (tf) antigen in normal, iugr, preeclamptic and hellp placentas. Placenta 2007, 28, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; Spaans, F.; de Vos, P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front. Immunol. 2014, 5, 298. [Google Scholar] [CrossRef] [PubMed]
- Ristich, V.; Liang, S.; Zhang, W.; Wu, J.; Horuzsko, A. Tolerization of dendritic cells by hla-g. Eur. J. Immunol. 2005, 35, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Bourne, Y.; Bolgiano, B.; Liao, D.I.; Strecker, G.; Cantau, P.; Herzberg, O.; Feizi, T.; Cambillau, C. Crosslinking of mammalian lectin (galectin-1) by complex biantennary saccharides. Nat. Struct. Biol. 1994, 1, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, N.; Ilarregui, J.M.; Toscano, M.A.; Rabinovich, G.A. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens 2004, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Gray, R.M.; Haudek, K.C.; Patterson, R.J. Nucleocytoplasmic lectins. Biochim. Biophys. Acta 2004, 1673, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Patterson, R.J.; Wang, J.L. Intracellular functions of galectins. Biochim. Biophys. Acta 2002, 1572, 263–273. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Sotomayor, C.E.; Riera, C.M.; Bianco, I.; Correa, S.G. Evidence of a role for galectin-1 in acute inflammation. Eur. J. Immunol. 2000, 30, 1331–1339. [Google Scholar] [CrossRef]
- Arata, Y.; Hirabayashi, J.; Kasai, K. Sugar binding properties of the two lectin domains of the tandem repeat-type galectin lec-1 (N32) of caenorhabditis elegans. Detailed analysis by an improved frontal affinity chromatography method. J. Biol. Chem. 2001, 276, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- Varela, P.F.; Romero, A.; Sanz, L.; Romao, M.J.; Topfer-Petersen, E.; Calvete, J.J. The 2.4 Å resolution crystal structure of boar seminal plasma PSP-I/PSP-II: A zona pellucida-binding glycoprotein heterodimer of the spermadhesin family built by a cub domain architecture. J. Mol. Biol. 1997, 274, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, Y. Purification of a 63 kDa β-d-galactoside binding lectin from cuttlefish, todarodes pacificus. Biochem. Mol. Biol. Int. 1997, 41, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, U.; Karsten, U.; Wiest, I.; Schulze, S.; Kuhn, C.; Friese, K.; Walzel, H. Binding of galectin-1 (gal-1) to the thomsen-friedenreich (TF) antigen on trophoblast cells and inhibition of proliferation of trophoblast tumor cells in vitro by gal-1 or an anti-tf antibody. Histochem. Cell Biol. 2006, 126, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, U.; Reimer, T.; Bergemann, C.; Wiest, I.; Schulze, S.; Friese, K.; Walzel, H. Binding of galectin-1 (gal-1) on trophoblast cells and inhibition of hormone production of trophoblast tumor cells in vitro by gal-1. Histochem. Cell Biol. 2004, 121, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.; Redel, S.; Hofmann, S.; Kuhn, C.; Friese, K.; Walzel, H.; Jeschke, U. Stimulation of syncytium formation in vitro in human trophoblast cells by galectin-1. Placenta 2010, 31, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Bozic, M.; Petronijevic, M.; Milenkovic, S.; Atanackovic, J.; Lazic, J.; Vicovac, L. Galectin-1 and galectin-3 in the trophoblast of the gestational trophoblastic disease. Placenta 2004, 25, 797–802. [Google Scholar] [PubMed]
- Jeschke, U.; Toth, B.; Scholz, C.; Friese, K.; Makrigiannakis, A. Glycoprotein and carbohydrate binding protein expression in the placenta in early pregnancy loss. J. Reprod. Immunol. 2010, 85, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ramhorst, R.E.; Giribaldi, L.; Fraccaroli, L.; Toscano, M.A.; Stupirski, J.C.; Romero, M.D.; Durand, E.S.; Rubinstein, N.; Blaschitz, A.; Sedlmayr, P.; et al. Galectin-1 confers immune privilege to human trophoblast: Implications in recurrent fetal loss. Glycobiology 2012, 22, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Vicovac, L.; Jankovic, M.; Cuperlovic, M. Galectin-1 and -3 in cells of the first trimester placental bed. Hum. Reprod. 1998, 13, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Ding, G.; Zhou, J.; Wang, H.; Zhao, Y.; Huang, H. The effect of trophoblasts on T lymphocytes: Possible regulatory effector molecules—A proteomic analysis. Cell. Physiol. Biochem.: Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2008, 21, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Karmakar, S.; Stowell, C.J.; Dias-Baruffi, M.; McEver, R.P.; Cummings, R.D. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood 2007, 109, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Lensch, M.; Andre, S.; Kaltner, H.; Wiedenmann, B.; Rosewicz, S.; Dignass, A.U.; Gabius, H.J. Human galectin-2: Novel inducer of t cell apoptosis with distinct profile of caspase activation. J. Immunol. 2004, 173, 3825–3837. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Inoue, K.; Sato, H.; Iida, A.; Ohnishi, Y.; Sekine, A.; Sato, H.; Odashiro, K.; Nobuyoshi, M.; Hori, M.; et al. Functional variation in LGALS2 confers risk of myocardial infarction and regulates lymphotoxin-alpha secretion in vitro. Nature 2004, 429, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Paclik, D.; Berndt, U.; Guzy, C.; Dankof, A.; Danese, S.; Holzloehner, P.; Rosewicz, S.; Wiedenmann, B.; Wittig, B.M.; Dignass, A.U.; et al. Galectin-2 induces apoptosis of lamina propria T lymphocytes and ameliorates acute and chronic experimental colitis in mice. J. Mol. Med. (Berlin, Germany) 2008, 86, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lei, C.; Zhang, W. Expression of galectin-3 in mouse endometrium and its effect during embryo implantation. Reprod. Biomed. Online 2012, 24, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Menkhorst, E.M.; Gamage, T.; Cuman, C.; Kaitu’u-Lino, T.J.; Tong, S.; Dimitriadis, E. Galectin-7 acts as an adhesion molecule during implantation and increased expression is associated with miscarriage. Placenta 2014, 35, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Yap, J.; Gamage, T.; Salamonsen, L.; Dimitriadis, E.; Menkhorst, E. Galectin-7 is important for normal uterine repair following menstruation. Mol. Hum. Reprod. 2014, 20, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Timmons, P.M.; Colnot, C.; Cail, I.; Poirier, F.; Magnaldo, T. Expression of galectin-7 during epithelial development coincides with the onset of stratification. Int. J. Dev. Biol. 1999, 43, 229–235. [Google Scholar] [PubMed]
- Menkhorst, E.; Koga, K.; van Sinderen, M.; Dimitriadis, E. Galectin-7 serum levels are altered prior to the onset of pre-eclampsia. Placenta 2014, 35, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Labrie, M.; Vladoiu, M.C.; Grosset, A.A.; Gaboury, L.; St-Pierre, Y. Expression and functions of galectin-7 in ovarian cancer. Oncotarget 2014, 5, 7705–7721. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Hiromasa, K.; Kabashima-Kubo, R.; Yoshioka, M.; Nakamura, M. Galectin-7, induced by cis-urocanic acid and ultraviolet b irradiation, down-modulates cytokine production by t lymphocytes. Exp. Dermatol. 2013, 22, 840–842. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Sumegi, B.; Than, G.N.; Berente, Z.; Bohn, H. Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13), a new lysophospholipase, homologue of human eosinophil charcot-leyden crystal protein. Placenta 1999, 20, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Abedin, M.J.; Kashio, Y.; Seki, M.; Nakamura, K.; Hirashima, M. Potential roles of galectins in myeloid differentiation into three different lineages. J. Leukoc. Biol. 2003, 73, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Kubach, J.; Lutter, P.; Bopp, T.; Stoll, S.; Becker, C.; Huter, E.; Richter, C.; Weingarten, P.; Warger, T.; Knop, J.; et al. Human CD4+CD25+ regulatory T cells: Proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood 2007, 110, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.C.; Won, K.J.; Jung, S.H.; Lee, K.P.; Lee, D.Y.; Park, E.S.; Kim, B.; Cheon, G.J.; Han, K.H. Proteomic analysis of colonic mucosal tissue from tuberculous and ulcerative colitis patients. Korean J. Physiol. Pharmacol. 2012, 16, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Agesen, T.H.; Berg, M.; Clancy, T.; Thiis-Evensen, E.; Cekaite, L.; Lind, G.E.; Nesland, J.M.; Bakka, A.; Mala, T.; Hauss, H.J.; et al. CLC and IFNAR1 are differentially expressed and a global immunity score is distinct between early- and late-onset colorectal cancer. Genes Immunity 2011, 12, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.C.; Douglass, J.A.; Gillman, A.; O’Hehir, R.E.; Meeusen, E.N. Galectin-10, a potential biomarker of eosinophilic airway inflammation. PLoS ONE 2012, 7, e42549. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, I.; Schiessl, B.; Jeschke, U.; Vogl, J.; Makrigiannakis, A.; Kuhn, C.; Schulze, S.; Kainer, F.; Friese, K. Expression of inhibin/activin subunits alpha (-α), betaA (-βA), and βB (-βB) in placental tissue of normal, preeclamptic, and hellp pregnancies. Endocr. Pathol. 2006, 17, 19–33. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
- Jeppesen, C.; Nielsen, P.E. Photofootprinting of drug-binding sites on DNA using diazo- and azido-9-aminoacridine derivatives. Eur. J. Biochem. 1989, 182, 437–444. [Google Scholar] [CrossRef] [PubMed]








| Characteristic | Normal Pregnancy n = 15 | SPA n = 9 | RSA n = 12 | p Value (Kruskal–Wallis Test) |
|---|---|---|---|---|
| maternal age (years) | 33.0 ± 6.7 (26–40) | 31.5 ± 8.8 (19–43) | 34.3 ± 4.6 (25–39) | 0.813 |
| gestational age (weeks) | 9.1 ± 1.6 (7–14) | 9.84 ± 1.4 (7–12) | 8.7 ± 2.2 (7–11) | 0.370 |
| gravidity | 3.2 ± 1.3 (1–6) | 2.2 ± 2.6 (1–9) | 2.9 ± 0.8 (2–4) | 0.077 |
| parity | 1.6 ± 0.7 (0–3) | 1.2 ± 2.6 (0–8) | 0.7 ± 1.8 (0–2) | 0.475 |
| Antigene | Clone | Species and Isotype | Concentration/Dilution | Source of Ab | Detection System | Positive Control Tissue |
|---|---|---|---|---|---|---|
| Gal-1 | K8508 | goat | 10 µg/mL 1:3000 in Power Block | R&D Systems, Minneapolis, MN, USA | Vectastain Elite Kit (Linaris, Dossenheim, Germany) | Colon, Mamma-Ca |
| Gal-2 | H-45 | rabbit | 1:500 in Dako diluting medium | Santa Cruz, Dallas, TX, USA | Zytochem-Plus HRP-Polymer-Kit (Mouse/Rabbit) (Zytomed, Berlin Germany) | Colon |
| Gal-3 | 9C4 | mouse | 4.6 mg/mL 1:1000 in PBS | Novocastra, Wetzlar, Germany | Vectastain Elite Kit (Linaris) | Colon |
| Gal-7 | H 60 | rabbit | 200 µg/mL 1:150 in Dako diluting medium | Santa Cruz | Zytochem-Plus HRP-Polymer-Kit (Mouse/Rabbit) (Zytomed) | Cervix |
| Gal-10 | H-40 | rabbit | 200 µg/mL 1:100 in Dako diluting medium | Santa Cruz | Zytochem-Plus HRP-Polymer-Kit (Mouse/Rabbit) (Zytomed) | Placenta |
| Primary Antibody | Secondary Antibody | |
|---|---|---|
| HLA-G, green | Mouse-IgG1 Clon MEM-6/9 (AbD Serotec, Puchheim, Germany) dilution 1:50 in Dako | Cy2-labeled Goat-Anti-Mouse IgG (Dianova Hamburg, Germany) diluted 1:100 in Dako → green |
| Gal 2, red | rabbit, (H-45) santa cruz | Cy3-labeled Goat-Anti-Rabbit IgG (Dianova) diluted 1:500 in Dako → red |
| Decidua | Villous Trophoblast | ||||
|---|---|---|---|---|---|
| p-value | gal-2 | gal-1 | gal-2 | gal-7 | gal-10 |
| p (IRS) | 0.016 | 0.006 | 0.001 | 0.006 | 0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unverdorben, L.; Haufe, T.; Santoso, L.; Hofmann, S.; Jeschke, U.; Hutter, S. Prototype and Chimera-Type Galectins in Placentas with Spontaneous and Recurrent Miscarriages. Int. J. Mol. Sci. 2016, 17, 644. https://doi.org/10.3390/ijms17050644
Unverdorben L, Haufe T, Santoso L, Hofmann S, Jeschke U, Hutter S. Prototype and Chimera-Type Galectins in Placentas with Spontaneous and Recurrent Miscarriages. International Journal of Molecular Sciences. 2016; 17(5):644. https://doi.org/10.3390/ijms17050644
Chicago/Turabian StyleUnverdorben, Laura, Thomas Haufe, Laura Santoso, Simone Hofmann, Udo Jeschke, and Stefan Hutter. 2016. "Prototype and Chimera-Type Galectins in Placentas with Spontaneous and Recurrent Miscarriages" International Journal of Molecular Sciences 17, no. 5: 644. https://doi.org/10.3390/ijms17050644
APA StyleUnverdorben, L., Haufe, T., Santoso, L., Hofmann, S., Jeschke, U., & Hutter, S. (2016). Prototype and Chimera-Type Galectins in Placentas with Spontaneous and Recurrent Miscarriages. International Journal of Molecular Sciences, 17(5), 644. https://doi.org/10.3390/ijms17050644