High DRC Levels Are Associated with Let-7b Overexpression in Women with Breast Cancer
Abstract
:1. Introduction
2. Results
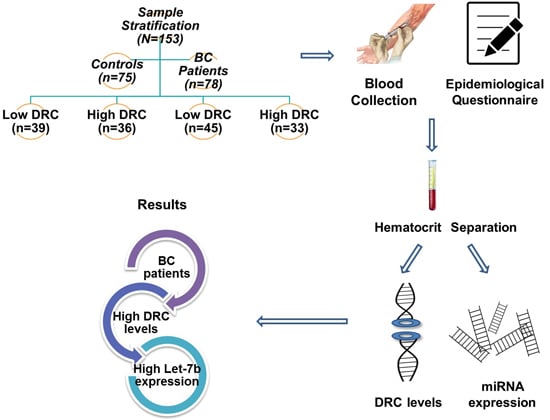
2.1. Sample Stratification
2.2. Demographic Characteristics of the Control and Breast Cancer Study Groups
2.3. Let-7b Expression
2.4. Correlations and Associations Analyses between Groups
2.5. Let-7b Results Based on Clinicopathological Characteristics
3. Discussion
3.1. Let-7 Expression
3.2. Epidemiological Associations
3.3. Clinicopathological Characteristics
4. Materials and Methods
4.1. Patient Recruitment
4.2. DNA Repair Capacity Measurements
4.3. Sample Stratification
4.4. mRNA Extraction and cDNA Synthesis
4.5. Let-7b Expression Using Real-Time PCR
4.6. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rougvie, A.E. Control of developmental timing in animals. Nat. Rev. Genet. 2001, 2, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Barh, D. Let-7 replacement therapy: Applicability in cancer. Cancer Ther. 2008, 6, 969–984. [Google Scholar]
- Slack, F.J.; Basson, M.; Liu, Z.; Ambros, V.; Horvitz, H.R.; Ruvkun, G. The lin-41 RBCC gene acts in the C. Elegans heterochronic pathway between the let-7 regulatory rna and the lin-29 transcription factor. Mol. Cell 2000, 5, 659–669. [Google Scholar] [CrossRef]
- Kumar, M.S.; Lu, J.; Mercer, K.L.; Golub, T.R.; Jacks, T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 2007, 39, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Barh, D.; Parida, S.; Parida, B.P.; Viswanathan, G. Let-7, miR-125, miR-205, and miR-296 are prospective therapeutic agents in breast cancer molecular medicine. Gene Ther. Mol. Biol. 2008, 12, 189–206. [Google Scholar]
- Soifer, H.S.; Rossi, J.J.; Saetrom, P. Micrornas in disease and potential therapeutic applications. Mol. Ther. J. Am. Soc. Gene Ther. 2007, 15, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. MiR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microrna biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, L.; Wang, Y.; Wang, X.; Liu, N.; You, Y. Regulation of Let-7 and its target oncogenes (review). Oncol. Lett. 2012, 3, 955–960. [Google Scholar] [PubMed]
- Joosse, S.; Müller, V.; Steinbach, B.; Pantel, K.; Schwarzenbach, H. Circulating cell-free cancer-testis MAGE-a RNA, BORIS RNA, Let-7b and miR-202 in the blood of patients with breast cancer and benign breast diseases. Br. J. Cancer 2014, 111, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Feuer, E.J.; Wun, L.-M.; Boring, C.C.; Flanders, W.D.; Timmel, M.J.; Tong, T. The lifetime risk of developing breast cancer. J. Natl. Cancer Inst. 1993, 85, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D. DNA Repair and Mutagenesis; American Society for Microbiology Press: Washington, DC, USA, 2005. [Google Scholar]
- Tessitore, A.; Cicciarelli, G.; del Vecchio, F.; Gaggiano, A.; Verzella, D.; Fischietti, M.; Vecchiotti, D.; Capece, D.; Zazzeroni, F.; Alesse, E. Micrornas in the DNA damage/repair network and cancer. Int. J. Genom. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.D.; Mitchell, M.; Sgouros, J.; Lindahl, T. Human DNA repair genes. Science 2001, 291, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.; Echenique, M.; Negron, E.; Morales, L.; Vargas, W.; Gaetan, F.S.; Lizardi, E.R.; Torres, A.; Rosado, J.O.; Bolaños, G. The association of DNA repair with breast cancer risk in women. A comparative observational study. BMC Cancer 2012, 12, 490. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.M.; Ruiz, A.; Colen, R.; Lopez, I.D.; Grossman, L.; Matta, J.L. DNA repair and breast carcinoma susceptibility in women. Cancer 2004, 100, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.R. DNA repair-deficient diseases, xeroderma pigmentosum, cockayne syndrome and trichothiodystrophy. Biochimie 2003, 85, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Latimer, J.J.; Johnson, J.M.; Kelly, C.M.; Miles, T.D.; Beaudry-Rodgers, K.A.; Lalanne, N.A.; Vogel, V.G.; Kanbour-Shakir, A.; Kelley, J.L.; Johnson, R.R. Nucleotide excision repair deficiency is intrinsic in sporadic stage i breast cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 21725–21730. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Lin, S.-Y. DNA damage and breast cancer. World J. Clin. Oncol. 2011, 2, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.-M.; Park, H.-J.; Kim, C.H.; Kim, I. The significance of microrna Let-7b, miR-30c, and miR-200c expression in breast cancers. Korean J. Pathol. 2011, 45, 354–360. [Google Scholar] [CrossRef]
- Mayr, C.; Hemann, M.T.; Bartel, D.P. Disrupting the pairing between Let-7 and HMGA2 enhances oncogenic transformation. Science 2007, 315, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Kasinski, A.L.; Slack, F.J. Micrornas en route to the clinic: Progress in validating and targeting micrornas for cancer therapy. Nat. Rev. Cancer 2011, 11, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Swarup, V.; Rajeswari, M.R. Circulating (cell-free) nucleic acids—A promising, non-invasive tool for early detection of several human diseases. FEBS Lett. 2007, 581, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Fleischhacker, M.; Schmidt, B. Circulating nucleic acids (CNAs) and cancer—A survey. Biochim. Biophys. Acta 2007, 1775, 181–232. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Guo, J.; Zheng, L.; Li, C.; Zheng, T.M.; Tanyi, J.L.; Liang, S.; Benedetto, C.; Mitidieri, M.; Katsaros, D.; et al. The heterochronic microrna Let-7 inhibits cell motility by regulating the genes in the actin cytoskeleton pathway in breast cancer. Mol. Cancer Res. 2013, 11, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Mathur, R.; Hu, X.; Zhang, X.; Lu, X. Mirna response to DNA damage. Trends Biochem. Sci. 2011, 36, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Simone, N.L.; Soule, B.P.; Ly, D.; Saleh, A.D.; Savage, J.E.; Degraff, W.; Cook, J.; Harris, C.C.; Gius, D.; Mitchell, J.B. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS ONE 2009, 4, e6377. [Google Scholar] [CrossRef] [PubMed]
- Smart, D.J.; Halicka, H.D.; Schmuck, G.; Traganos, F.; Darzynkiewicz, Z.; Williams, G.M. Assessment of DNA double-strand breaks and γH2AX induced by the topoisomerase II poisons etoposide and mitoxantrone. Mutat. Res. 2008, 641, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, K.; Low, G.K.; Ting, A.P.; Srikanth, P.; Slijepcevic, P.; Hande, M.P. Hydrogen peroxide induced genomic instability in nucleotide excision repair-deficient lymphoblastoid cells. Genome Integr. 2010, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.A. Base excision repair of ionizing radiation-induced DNA damage in G1 and G2 cell cycle phases. Cancer Cell Int. 2007, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Fournie, G.J.; Courtin, J.P.; Laval, F.; Chale, J.J.; Pourrat, J.P.; Pujazon, M.C.; Lauque, D.; Carles, P. Plasma DNA as a marker of cancerous cell death. Investigations in patients suffering from lung cancer and in nude mice bearing human tumours. Cancer Lett. 1995, 91, 221–227. [Google Scholar] [CrossRef]
- Giacona, M.B.; Ruben, G.C.; Iczkowski, K.A.; Roos, T.B.; Porter, D.M.; Sorenson, G.D. Cell-free DNA in human blood plasma: Length measurements in patients with pancreatic cancer and healthy controls. Pancreas 1998, 17, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N. Serum micrornas are promising novel biomarkers. PLoS ONE 2008, 3, e3148. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, G.; Comte, D.; Leon, M.; Ciricione, R.; Roz, L.; Ratcliffe, C.; Roz, E.; Cirenei, N.; Bellomi, M.; Pelosi, G.; et al. Quantification of free circulating DNA as a diagnostic marker in lung cancer. J. Clin. Oncol. 2003, 21, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Angelillo-Scherrer, A. Leukocyte-derived microparticles in vascular homeostasis. Circ. Res. 2012, 110, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.; Tein, M.S.; Lau, T.K.; Haines, C.J.; Leung, T.N.; Poon, P.M.; Wainscoat, J.S.; Johnson, P.J.; Chang, A.M.; Hjelm, N.M. Quantitative analysis of fetal DNA in maternal plasma and serum: Implications for noninvasive prenatal diagnosis. Am. J. Hum. Genet. 1998, 62, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, J.L.; Gammon, M.D.; John, E.M. Reproductive factors and breast cancer. Epidemiol. Rev. 1993, 15, 36. [Google Scholar] [PubMed]
- Lord, S.J.; Bernstein, L.; Johnson, K.A.; Malone, K.E.; McDonald, J.A.; Marchbanks, P.A.; Simon, M.S.; Strom, B.L.; Press, M.F.; Folger, S.G. Breast cancer risk and hormone receptor status in older women by parity, age of first birth, and breastfeeding: A case-control study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Miller, B. Cancer: We Can Win the War against Cancer by Aggresively Pursuing Prevention; Oak Publication Sdn Bhd: Petaling Jaya, Malaysia, 2005. [Google Scholar]
- Cerhan, J.R. Oral contraceptive use and breast cancer risk: Current status. Mayo Clin. Proc. 2006, 81, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Modugno, F.; Ness, R.B.; Allen, G.O.; Schildkraut, J.M.; Davis, F.G.; Goodman, M.T. Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis. Am. J. Obstet. Gynecol. 2004, 191, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Kahlenborn, C.; Modugno, F.; Potter, D.M.; Severs, W.B. Oral contraceptive use as a risk factor for premenopausal breast cancer: A meta-analysis. Mayo Clin. Proc. 2006, 81, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A.; Grossardt, B.R.; de Andrade, M.; Malkasian, G.D.; Melton Iii, L.J. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006, 7, 821–828. [Google Scholar] [CrossRef]
- Olson, J.E.; Sellers, T.A.; Iturria, S.J.; Hartmann, L.C. Bilateral oophorectomy and breast cancer risk reduction among women with a family history. Cancer Detect. Prev. 2004, 28, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.A. Oophorectomy for breast cancer prevention in women with BRCA1 or BRCA2 mutations. Women’s Health 2009, 5, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Million Women Study, C. Breast cancer and hormone-replacement therapy in the million women study. Lancet 2003, 362, 419–427. [Google Scholar]
- Mao, X.; He, J.; Li, T.; Lu, Z.; Sun, J.; Meng, Y.; Abliz, Z.; Chen, J. Application of imaging mass spectrometry for the molecular diagnosis of human breast tumors. Sci. Rep. 2016, 6, 21043. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. [Google Scholar] [CrossRef] [PubMed]
- Arsuaga, J.; Borrman, T.; Cavalcante, R.; Gonzalez, G.; Park, C. Identification of copy number aberrations in breast cancer subtypes using persistence topology. Microarrays 2015, 4, 339. [Google Scholar] [CrossRef]
- Matta, J.; Morales, L.; Ortiz, C.; Adams, D.; Vargas, W.; Casbas, P.; Dutil, J.; Echenique, M.; Suarez, E. Estrogen receptor expression is associated with DNA repair capacity in breast cancer. PLoS ONE 2016, 11, e0152422. [Google Scholar] [CrossRef] [PubMed]
- Kroh, E.M.; Parkin, R.K.; Mitchell, P.S.; Tewari, M. Analysis of circulating microrna biomarkers in plasma and serum using quantitative reverse transcription-PCR (QRT-PCR). Methods 2010, 50, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Athas, W.F.; Hedayati, M.A.; Matanoski, G.M.; Farmer, E.R.; Grossman, L. Development and field-test validation of an assay for DNA repair in circulating human lymphocytes. Cancer Res. 1991, 51, 5786–5793. [Google Scholar] [PubMed]
- Kirschner, M.B.; Kao, S.C.; Edelman, J.J.; Armstrong, N.J.; Vallely, M.P.; van Zandwijk, N.; Reid, G. Haemolysis during sample preparation alters microrna content of plasma. PLoS ONE 2011, 6, e24145. [Google Scholar] [CrossRef] [PubMed]



| Variable | Number of Patients (%) | p Value | |
|---|---|---|---|
| BC Group (n = 78) | Control Group (n = 75) | ||
| DRC | |||
| Low (<3.8) | 43 (28) | 36 (24.0) | |
| High (≥3.8) | 35 (23.0) | 39 (25) | 0.4714 |
| Age | |||
| 21–40 years | 8 (10.2) | 13 (17.3) | |
| 41–60 years | 34 (43.6) | 46 (61.3) | 0.0049 |
| ≥ 61 years | 36 (46.2) | 16 (21.3) | |
| Body mass index | |||
| <25 kg/m2 | 24 (30.8) | 30 (40.0) | |
| ≥25 kg/m2 | 53 (67.9) | 42 (56.0) | 0.4045 |
| Missing | 1 (1.3) | 3 (4.0) | |
| Ever been pregnant | |||
| Yes | 69 (88.5) | 60 (80.0) | |
| No | 9 (11.5) | 15 (20.0) | 0.2238 |
| Age at first birth | |||
| ≤19 years | 21 (30.4) | 9 (15.0) | |
| 20–29 years | 33 (47.8) | 47 (78.3) | |
| ≥30 years | 13 (18.8) | 4 (6.7) | 0.0029 |
| Missing | 2 (2.9) | 0 (0.0) | |
| Ever breastfeed | |||
| Yes | 47 (68.1) | 41 (68.3) | |
| No | 22 (31.9) | 19 (31.7) | <0.9789 |
| Oral contraceptive use | |||
| Yes | 33 (42.3) | 37 (49.3) | 0.4779 |
| No | 45 (57.7) | 38 (50.7) | |
| Age started oral contraceptive | |||
| <20 years | 4 (12.1) | 9 (24.3) | |
| ≥21 years | 26 (78.8) | 27 (73.0) | 0.3811 |
| Missing | 3 (9.1) | 1 (2.7) | |
| Regular menstrual periods | |||
| Yes | 52 (66.7) | 39 (52.0) | |
| No | 26 (33.3) | 35 (46.7) | 0.1118 |
| Missing | 0 (0.0) | 1 (1.3) | |
| Age at menarche | |||
| ≤12 years | 40 (51.3) | 40 (53.3) | |
| ≥13 years | 37 (47.4) | 35 (46.7) | 0.9932 |
| Missing | 1 (1.3) | 0 (0.0) | |
| History of endometriosis | |||
| Yes | 5 (6.4) | 4 (5.3) | |
| No | 73 (93.6) | 71 (94.7) | 0.7772 |
| Hysterectomy | |||
| Yes | 19 (24.4) | 17 (22.7) | |
| No | 59 (75.6) | 58 (77.3) | 0.9553 |
| Age of hysterectomy | |||
| ≤40 years | 6 (31.6) | 7 (41.2) | |
| 41–49 years | 10 (52.6) | 10 (58.8) | |
| ≥50 years | 3 (15.8) | 0 (0.0) | 0.2259 |
| Oophorectomy | |||
| Yes | 12 (15.4) | 15 (20.0) | |
| No | 66 (84.6) | 59 (78.7) | |
| Missing | 0 (0.0) | 1 (1.3) | 0.5255 |
| Age of oophorectomy | |||
| ≤40 years | 0 (0.0) | 6 (40.0) | |
| 41–49 years | 4 (33.3) | 4 (26.7) | |
| ≥50 years | 6 (50.0) | 3 (20.0) | 0.0347 |
| Missing | 2 (16.7) | 2 (13.3) | |
| Menopause | |||
| Yes | 60 (76.9) | 53 (70.7) | |
| No | 18 (23.1) | 22 (29.3) | 0.4623 |
| Hormone replacement therapy | |||
| Yes | 17 (21.8) | 31 (41.3) | |
| No | 61 (78.2) | 44 (58.7) | 0.0143 |
| Smoking | |||
| Yes | 13 (16.7) | 7 (9.3) | |
| No | 65 (83.3) | 68 (90.7) | 0.232 |
| Alcohol consumption | |||
| Yes | 15 (19.2) | 14 (18.7) | |
| No | 63 (80.8) | 61 (81.3) | 1 |
| Family history of cancer (not BC) | |||
| Yes | 49 (62.8) | 38 (50.7) | |
| No | 29 (37.2) | 37 (49.3) | 0.4623 |
| BC history in any family member | |||
| Yes | 23 (29.5) | 14 (18.7) | |
| No | 55 (70.5) | 61 (81.3) | 0.1339 |
| Variable | Number of BC Patients (%) | Let-7b 1 p-Value | |
|---|---|---|---|
| Low DRC (<3.8) Group (n = 43) | High DRC (≥3.8) Group (n = 33) | ||
| Estrogen receptor | |||
| Positive | 34 (79.1) | 20 (60.6) | 0.0007 |
| Negative | 4 (9.3) | 7 (21.2) | p ˃ 0.05 |
| Missing | 5 (11.6) | 6 (18.2) | |
| Progesterone receptor | |||
| Positive | 30 (69.8) | 18 (54.5) | 0.0007 |
| Negative | 8 (18.6) | 9 (27.3) | p ˃ 0.05 |
| Missing | 5 (11.6) | 6 (18.2) | |
| HER2 | |||
| Positive | 7 (16.3) | 9 (27.3) | p ˃ 0.05 |
| Negative | 23 (53.5) | 16 (48.5) | 0.0258 |
| Missing | 13 (30.2) | 8 (24.2) | |
| Grade | |||
| I | 6 (14.0) | 2 (6.1) | p ˃ 0.05 |
| II | 19 (44.2) | 15 (45.5) | p ˃ 0.05 |
| III | 7 (16.3) | 8 (24.2) | p ˃ 0.05 |
| Missing | 11 (25.6) | 8 (24.2) | |
| Subtypes | |||
| Luminal A | 23 (53.5) | 14 (42.4) | 0.0063 |
| Luminal B | 7 (16.3) | 9 (27.3) | p ˃ 0.05 |
| Triple negative | 1 (2.3) | 2 (6.1) | p ˃ 0.05 |
| Missing | 12 (27.9) | 8 (24.2) | |
| Site | |||
| Ductal | 39 (90.7) | 28 (84.8) | 0.0140 |
| Ductal + lobular | 0 (0.0) | 1 (3.0) | p ˃ 0.05 |
| Lobular | 2 (4.7) | 2 (6.1) | p ˃ 0.05 |
| Missing | 2 (4.7) | 2 (6.1) | |
| Type | |||
| In situ | 4 (9.3) | 6 (18.2) | 0.0019 |
| Invasive | 38 (88.4) | 27 (81.8) | p ˃ 0.05 |
| Missing | 1 (2.3) | 0 (0.0) | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encarnación, J.; Ortiz, C.; Vergne, R.; Vargas, W.; Coppola, D.; Matta, J.L. High DRC Levels Are Associated with Let-7b Overexpression in Women with Breast Cancer. Int. J. Mol. Sci. 2016, 17, 865. https://doi.org/10.3390/ijms17060865
Encarnación J, Ortiz C, Vergne R, Vargas W, Coppola D, Matta JL. High DRC Levels Are Associated with Let-7b Overexpression in Women with Breast Cancer. International Journal of Molecular Sciences. 2016; 17(6):865. https://doi.org/10.3390/ijms17060865
Chicago/Turabian StyleEncarnación, Jarline, Carmen Ortiz, Ralphdy Vergne, Wanda Vargas, Domenico Coppola, and Jaime L. Matta. 2016. "High DRC Levels Are Associated with Let-7b Overexpression in Women with Breast Cancer" International Journal of Molecular Sciences 17, no. 6: 865. https://doi.org/10.3390/ijms17060865
APA StyleEncarnación, J., Ortiz, C., Vergne, R., Vargas, W., Coppola, D., & Matta, J. L. (2016). High DRC Levels Are Associated with Let-7b Overexpression in Women with Breast Cancer. International Journal of Molecular Sciences, 17(6), 865. https://doi.org/10.3390/ijms17060865