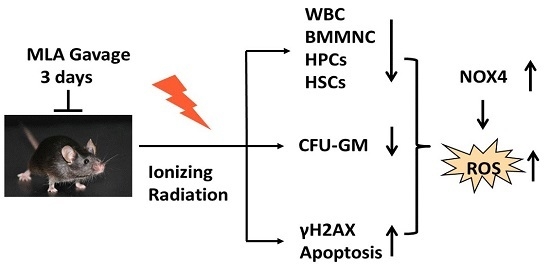
The Protective Effects of 5-Methoxytryptamine-α-lipoic Acid on Ionizing Radiation-Induced Hematopoietic Injury
Abstract
:1. Introduction
2. Results
2.1. Thirty-Day Survival after Exposure to 7.2 Gy Total Body Irradiation (TBI)
2.2. 5-Methoxytryptamine-α-lipoic Acid (MLA) Elevated Peripheral Blood Cell Counts after Exposure to 6 Gy TBI
2.3. The Effect of MLA on the Colony Form Unit Granulocyte and Monocyte (CFU-GM) Frequency and the Number of CFU-S after Exposure to 6 Gy TBI
2.4. The Effect of MLA on BM Mononuclear Cell (BMMNC) Apoptosis after Exposure to 6 Gy TBI
2.5. The Effects of MLA on Hematopoietic DNA Injury after Exposure to 6 Gy TBI
2.6. The Effects of MLA on Reactive Oxygen Species(ROS) Levels in Hematopoietic Cells after Exposure to 6 Gy TBI
2.7. The Effects of MLA on the Expression of NOX4 in Hematopoietic Cells after Exposure to 6 Gy TBI
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Reagents
4.3. Animals
4.4. Irradiation and Treatment
4.5. Peripheral Blood Cell and BMMNC Counts
4.6. Detection of Hematopoietic Progenitor Cells (HPCs) and Hematopoietic Stem Cells (HSCs)
4.7. Colony-Forming Cell (CFC) Assay
4.8. Endogenous Spleen Colony-Forming Units
4.9. Intracellular ROS Analysis
4.10. Analysis of H2AX Phosphorylation and NOX4 Expression
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, C.; Zhang, B.; Wang, S.; Zhang, J.; Liu, Y.; Wang, J.; Fan, Z.; Lv, Y.; Zhang, X.; He, L.; et al. Recombinant human thrombopoietin promotes hematopoietic reconstruction after severe whole body irradiation. Sci. Rep. 2015, 5, 12993. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular pathways in response to ionizing radiation and their targetability for tumor radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Johnke, R.M.; Sattler, J.A.; Allison, R.R. Radioprotective agents for radiation therapy: Future trends. Future Oncol. 2014, 10, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Hosseinimehr, S.J. Trends in the development of radioprotective agents. Drug Discov. Today 2007, 12, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.M.; Day, R.; Singh, V.K. New approaches to radiation protection. Front. Oncol. 2014, 4, 381. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev. 1980, 1, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi; Reiter, R.J.; Herman, T.S.; Meltz, M.L. Melatonin and radioprotection from genetic damage: In vivo/in vitro studies with human volunteers. Mutat. Res. 1996, 371, 221–228. [Google Scholar] [CrossRef]
- Packer, L.; Witt, E.H.; Tritschler, H.J. α-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Cudkowicz, G.; Franceschini, J. α-Lipoic acid and chemical protection against ionizing radiation. Arch. Int. Pharmacodyn. Ther. 1959, 122, 312–317. [Google Scholar] [PubMed]
- Koufaki, M. Therapeutic applications of lipoic acid: A patent review (2011–2014). Expert. Opin. Ther. Pat. 2014, 24, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Mihandoost, E.; Shirazi, A.; Mahdavi, S.R.; Aliasgharzadeh, A. Consequences of lethal-whole-body gamma radiation and possible ameliorative role of melatonin. Sci. World J. 2014, 2014, 621570. [Google Scholar] [CrossRef] [PubMed]
- Karaer, I.; Simsek, G.; Gul, M.; Bahar, L.; Gurocak, S.; Parlakpinar, H.; Nuransoy, A. Melatonin protects inner ear against radiation damage in rats. Laryngoscope 2015, 125, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Liu, J.; Shi, J.; Zhou, Q.; Zhang, X.; Du, Z.; Liu, Q.; Guo, Y. Melatonin protects ADSCs from ROS and enhances their therapeutic potency in a rat model of myocardial infarction. J. Cell. Mol. Med. 2015, 19, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Wu, H.; Lu, L.; Wang, X.; Zhang, J.; Zhang, H.; Fan, S.; Fan, F.; Zhou, D.; et al. The effects of p38 MAPK inhibition combined with G-CSF administration on the hematoimmune system in mice with irradiation injury. PLoS ONE 2013, 8, e62921. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lu, L.; Zhang, J.; Wang, X.; Xing, Y.; Wu, H.; Yang, X.; Shi, Z.; Zhao, M.; Fan, S.; et al. Mitigating the effects of Xuebijing injection on hematopoietic cell injury induced by total body irradiation with gamma rays by decreasing reactive oxygen species levels. Int. J. Mol. Sci. 2014, 15, 10541–10553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Pazhanisamy, S.K.; Li, H.; Meng, A.; Zhou, D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic. Biol. Med. 2010, 48, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Drouet, M.; Herodin, F. Radiation victim management and the haematologist in the future: Time to revisit therapeutic guidelines? Int. J. Radiat. Biol. 2010, 86, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, J.; Xu, W.; Qi, Y.; Lu, Y.; Qiu, L.; Hu, Z.; Chu, Z.; Chai, Y.; Zhang, J. Protective Effects of Hong Shan capsule against lethal total-body irradiation-Induced damage in wistar rats. Int. J. Mol. Sci. 2015, 16, 18938–18955. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Adhikari, J.S.; Rizvi, M.A.; Chaudhury, N.K. Radioprotective potential of melatonin against 60Co gamma-ray-induced testicular injury in male C57BL/6 mice. J. Biomed. Sci. 2015, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, R.; Shabestani Monfared, A.; Tahamtani, Y.; Tavassoli, A.; Akmali, M.; Mosleh-Shirazi, M.A.; Naghizadeh, M.M.; Ghasemi, D.; Keshavarz, M.; Haddadi, G.H. Radioprotective effect of melatonin on radiation-induced lung injury and lipid peroxidation in rats. Cell. J. 2015, 17, 111–120. [Google Scholar] [PubMed]
- Sharma, S.; Haldar, C.; Chaube, S.K. Effect of exogenous melatonin on X-ray induced cellular toxicity in lymphatic tissue of Indian tropical male squirrel, Funambulus pennanti. Int. J. Radiat. Biol. 2008, 84, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, A.; Mihandoost, E.; Ghobadi, G.; Mohseni, M.; Ghazi-Khansari, M. Evaluation of radio-protective effect of melatonin on whole body irradiation induced liver tissue damage. Cell J. 2013, 14, 292–297. [Google Scholar] [PubMed]
- Shirazi, A.; Mihandoost, E.; Mohseni, M.; Ghazi-Khansari, M.; Rabie Mahdavi, S. Radio-protective effects of melatonin against irradiation-induced oxidative damage in rat peripheral blood. Phys. Med. 2013, 29, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Manchester, L.C. The disaster in Japan: Utility of melatonin in providing protection against ionizing radiation. J. Pineal Res. 2011, 50, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Karunakaran, U.; Jeoung, N.H.; Jeon, J.H.; Lee, I.K. Physiological effect and therapeutic application of alpha lipoic acid. Curr. Med. Chem. 2014, 21, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Jung, J.; Kim, S.K.; Woo, S.H.; Kang, K.M.; Jeong, B.K.; Jung, M.H.; Kim, J.H.; Hahm, J.R. α Lipoic acid attenuates radiation-induced thyroid injury in rats. PLoS ONE 2014, 9, e112253. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, L.; Nair, C.K. Protection against genotoxic damages following whole body γ radiation exposure in mice by lipoic acid. Mutat. Res. 2011, 724, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi; Meltz, M.L.; Reiter, R.J.; Herman, T.S.; Kumar, K.S. Melatonin and protection from whole-body irradiation: Survival studies in mice. Mutat. Res. 1999, 425, 21–27. [Google Scholar] [CrossRef]
- Kindekov, I.; Mileva, M.; Krastev, D.; Vassilieva, V.; Raynova, Y.; Doumanova, L.; Aljakov, M.; Idakieva, K. Radioprotective effect of hemocyanin in gamma induced acute radiation syndrome. Biotechnol. Biotechnol. Equip. 2014, 28, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Dainiak, N. Hematologic consequences of exposure to ionizing radiation. Exp. Hematol. 2002, 30, 513–528. [Google Scholar] [CrossRef]
- Shao, L.; Luo, Y.; Zhou, D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid Redox Signal. 2014, 20, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Kumar, A.; Adhikari, J.S.; Rizvi, M.A.; Chaudhury, N.K. Protective effect of sesamol against 60Co gamma-ray-induced hematopoietic and gastrointestinal injury in C57BL/6 male mice. Free Radic. Res. 2015, 49, 1344–1361. [Google Scholar] [CrossRef] [PubMed]
- Pirayesh Islamian, J.; Mehrali, H. Lycopene as a carotenoid provides radioprotectant and antioxidant effects by quenching radiation-induced free radical singlet oxygen: An overview. Cell J. 2015, 16, 386–391. [Google Scholar] [PubMed]
- Singh, P.K.; Krishnan, S. Vitamin E analogs as radiation response modifiers. Evid. Based Complement. Altern. Med. 2015, 2015, 741301. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, S.; Sharma, D.; Checker, R.; Thoh, M.; Gota, V.; Sandur, S.K.; Sainis, K.B. Amelioration of radiation-induced hematopoietic syndrome by an antioxidant chlorophyllin through increased stem cell activity and modulation of hematopoiesis. Free Radic. Biol. Med. 2015, 85, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Li, D.G.; Zhang, J.L.; Wu, H.Y.; Lu, L.; Wang, X.C.; Meng, A.M. Effects of d-methionine on the hematopoietic system injury in irradiated mice. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2014, 22, 421–424. (In Chinese) [Google Scholar] [PubMed]
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.R.; Dias, I.H.; Willetts, R.S.; Devitt, A. Redox regulation of protein damage in plasma. Redox Biol. 2014, 2, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Andrais, B.; Scott, A.; Wang, Y.W.; Murray, D. Ionizing radiation-induced responses in human cells with differing TP53 status. Int. J. Mol. Sci. 2013, 14, 22409–22435. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Fasth, A.; Ek, T.; Hammarsten, O. Validation of a flow cytometry-based detection of γ-H2AX, to measure DNA damage for clinical applications. Cytometry B Clin. Cytom. 2016. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, C.; Ria, R.; Scrima, R.; Cela, O.; D′Aprile, A.; Boffoli, D.; Falzetti, F.; Tabilio, A.; Capitanio, N. Characterization of mitochondrial and extra-mitochondrial oxygen consuming reactions in human hematopoietic stem cells. Novel evidence of the occurrence of NAD(P)H oxidase activity. J. Biol. Chem. 2005, 280, 26467–26476. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, C.; D′Aprile, A.; Ripoli, M.; Scrima, R.; Lecce, L.; Boffoli, D.; Tabilio, A.; Capitanio, N. Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem. Biophys. Res. Commun. 2007, 353, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wu, H.; Zhang, J.; Li, D.; Wang, Y.; Zhang, H.; Lu, L.; Li, C.; Huang, S.; Xing, Y.; et al. Metformin ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med. 2015, 87, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Wu, H.; Lu, L.; Zhang, H.; Chang, J.; Zhai, Z.; Zhang, J.; Zhou, D.; Meng, A. Mitigation of ionizing radiation-induced bone marrow suppression by p38 inhibition and G-CSF administration. J. Radiat. Res. 2011, 52, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Li, D.; Cao, X.; Zhang, Y.; Mu, J.; Lu, W.; Xiao, X.; Li, C.; Meng, J.; Chen, J.; et al. ROS-mediated iron overload injures the hematopoiesis of bone marrow by damaging hematopoietic stem/progenitor cells in mice. Sci. Rep. 2015, 5, 10181. [Google Scholar] [CrossRef] [PubMed]
- Till, J.E.; Mc Culloch, E.A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 1961, 14, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, F.; Shen, X.; Fan, S.; Liu, Q.; Wang, D. Polysaccharide isolated from Parmelia tinctorum ameliorates ionizing irradiation-induced damage in mice. J. Radiat. Res. 2014, 55, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Li, C.; Li, D.; Lu, L.; Wang, X.; Chang, J.; et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med. 2013, 54, 40–50. [Google Scholar] [CrossRef] [PubMed]










© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Tian, Z.; Tang, W.; Zhang, J.; Lu, L.; Sun, Z.; Zhou, Z.; Fan, F. The Protective Effects of 5-Methoxytryptamine-α-lipoic Acid on Ionizing Radiation-Induced Hematopoietic Injury. Int. J. Mol. Sci. 2016, 17, 935. https://doi.org/10.3390/ijms17060935
Li D, Tian Z, Tang W, Zhang J, Lu L, Sun Z, Zhou Z, Fan F. The Protective Effects of 5-Methoxytryptamine-α-lipoic Acid on Ionizing Radiation-Induced Hematopoietic Injury. International Journal of Molecular Sciences. 2016; 17(6):935. https://doi.org/10.3390/ijms17060935
Chicago/Turabian StyleLi, Deguan, Zhenyuan Tian, Weisheng Tang, Junling Zhang, Lu Lu, Zhaojin Sun, Zewei Zhou, and Feiyue Fan. 2016. "The Protective Effects of 5-Methoxytryptamine-α-lipoic Acid on Ionizing Radiation-Induced Hematopoietic Injury" International Journal of Molecular Sciences 17, no. 6: 935. https://doi.org/10.3390/ijms17060935
APA StyleLi, D., Tian, Z., Tang, W., Zhang, J., Lu, L., Sun, Z., Zhou, Z., & Fan, F. (2016). The Protective Effects of 5-Methoxytryptamine-α-lipoic Acid on Ionizing Radiation-Induced Hematopoietic Injury. International Journal of Molecular Sciences, 17(6), 935. https://doi.org/10.3390/ijms17060935