Voltage-Activated Calcium Channels as Functional Markers of Mature Neurons in Human Olfactory Neuroepithelial Cells: Implications for the Study of Neurodevelopment in Neuropsychiatric Disorders
Abstract
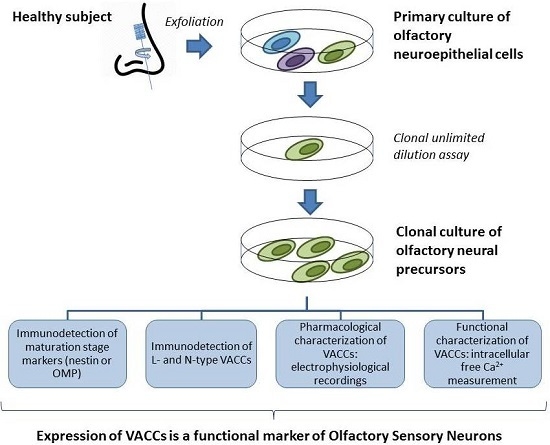
:1. Introduction
2. Results
2.1. Antibody Characterization by Western Blot
2.2. Immunodetection of OSN, Precursor Cells and VACC
2.3. Forskolin Induced an Increase in the Concentration of Intracellular Ca2+
2.4. Electrophysiological Recording of VACC-Dependent Currents
3. Discussion
4. Materials and Methods
4.1. Enriched Culture of Human Olfactory Precursor Cells
4.2. Antibody Characterization by Western Blot
4.3. Detection of Cellular Stage and VACC by Immunofluorescence
4.4. Detection of Intracellular Ca2+ by Microfluorometry
4.5. Electrophysiological Recording of VACC
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allen, K.M.; Fung, S.J.; Shannon Weickert, C. Cell proliferation is reduced in the hippocampus in schizophrenia. Aust. N. Z. J. Psychiatry 2015. [Google Scholar] [CrossRef] [PubMed]
- Reif, A.; Fritzen, S.; Finger, M.; Strobel, A.; Lauer, M.; Schmitt, A.; Lesch, K.P. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry 2006, 11, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, H.; Takao, K.; Walton, N.M.; Matsumoto, M.; Miyakawa, T. Immature dentate gyrus: An endophenotype of neuropsychiatric disorders. Neural Plast. 2013, 2013, 318596. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.M.; Zhou, Y.; Kogan, J.H.; Shin, R.; Webster, M.; Gross, A.K.; Heusner, C.L.; Chen, Q.; Miyake, S.; Tajinda, K.; et al. Detection of an immature dentate gyrus feature in human schizophrenia/bipolar patients. Transl. Psychiatry 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Han, L.Y.; Moberg, P.J.; Turetsky, B.I.; Gur, R.E.; Trojanowski, J.Q.; Hahn, C.G. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch. Gen. Psychiatry 2001, 58, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Moberg, P.J.; Arnold, S.E.; Doty, R.L.; Gur, R.E.; Balderston, C.C.; Roalf, D.R.; Gur, R.C.; Kohler, C.G.; Kanes, S.J.; Siegel, S.J.; et al. Olfactory functioning in schizophrenia: Relationship to clinical, neuropsychological and volumetric MRI measures. J. Clin. Exp. Neuropsychol. 2006, 28, 1444–1461. [Google Scholar] [CrossRef] [PubMed]
- Schild, D.; Restrepo, D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol. Rev. 1998, 78, 429–466. [Google Scholar] [PubMed]
- Benítez-King, G.; Riquelme, A.; Ortíz-López, L.; Berlanga, C.; Rodríguez-Verdugo, M.S.; Romo, F.; Calixto, E.; Solís-Chagoyán, H.; Jímenez, M.; Montaño, L.M.; et al. A non-invasive method to isolate the neuronal linage from the nasal epithelium from schizophrenic and bipolar diseases. J. Neurosci. Methods 2011, 201, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Low, V.F.; Faull, R.L. Neurogenesis and progenitor cells in the adult human brain: A comparison between hippocampal and subventricular progenitor proliferation. Dev. Neurobiol. 2012, 72, 990–1005. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Gheusi, G.; Lledo, P.M. Adult neurogenesis in the olfactory system shapes odor memory and perception. Prog. Brain Res. 2014, 208, 157–175. [Google Scholar] [PubMed]
- Zhang, X.; Klueber, K.M.; Guo, Z.; Cai, J.; Lu, C.; Winstead, W.I.; Qiu, M.; Roisen, F.J. Induction of neuronal differentiation of adult human olfactory neuroepithelial-derived progenitors. Brain Res. 2006, 1073–1074, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.A.; Tetzlaff, W.; Weiss, S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992, 12, 4565–4574. [Google Scholar] [PubMed]
- Cave, J.W.; Wang, M.; Baker, H. Adult subventricular zone neural stem cells as a potential source of dopaminergic replacement neurons. Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Lepski, G.; Jannes, C.E.; Nikkhah, G.; Bischofberger, J. cAMP promotes the differentiation of neural progenitor cells in vitro via modulation of voltage-gated calcium channels. Front. Cell Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Hogg, R.C.; Chipperfield, H.; Whyte, K.A.; Stafford, M.R.; Hansen, M.A.; Cool, S.M.; Nurcombe, V.; Adams, D.J. Functional maturation of isolated neural progenitor cells from the adult rat hippocampus. Eur. J. Neurosci. 2004, 19, 2410–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, S.; Rasika, S.; Han, J.; Mauduit, C.; Raccurt, M.; Morel, G.; Jourdan, F.; Benahmed, M.; Moyse, E.; Patterson, P.H. Leukemia inhibitory factor is a key signal for injury-induced neurogenesis in the adult mouse olfactory epithelium. J. Neurosci. 2003, 23, 1792–1803. [Google Scholar] [PubMed]
- Tucker, E.S.; Lehtinen, M.K.; Maynard, T.; Zirlinger, M.; Dulac, C.; Rawson, N.; Pevny, L.; Lamantia, A.S. Proliferative and transcriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium. Development 2010, 137, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Iwatsuki, K.; Ishihara, M.; Shikina, T.; Shinzawa, K.; Moriwaki, T.; Ninomiya, K.; Ohkawa, T.; Umegaki, M.; Kishima, H.; et al. Isolation of human adult olfactory sphere cells as a cell source of neural progenitors. Stem Cell Res. 2015, 15, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Rawson, N.E.; Ozdener, M.H. Primary culture of the human olfactory neuroepithelium. Methods Mol. Biol. 2013, 945, 81–93. [Google Scholar] [PubMed]
- Jafek, B.W.; Murrow, B.; Michaels, R.; Restrepo, D.; Linschoten, M. Biopsies of human olfactory epithelium. Chem. Sens. 2002, 27, 623–628. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Weiss, S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 1996, 175, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Marangoudakis, S.; Andrade, A.; Helton, T.D.; Denome, S.; Castiglioni, A.J.; Lipscombe, D. Differential ubiquitination and proteasome regulation of CaV2.2 N-type channel splice isoforms. J. Neurosci. 2012, 32, 10365–10369. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Maeshima, A.; Miya, M.; Sakurai, N.; Ikeuchi, H.; Hiromura, K.; Nojima, Y. Involvement of N-type Ca2+ channels in the fibrotic process of the kidney in rats. Am. J. Physiol. Ren. Physiol. 2013, 304, F665–F673. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Yu, D.; Hu, Z.; Liang, M.C.; Wang, J.J.; Yu, C.Y.; Ng, G.; Yong, T.F.; Soon, J.L.; Chua, Y.L.; et al. Alternative splicing generates a novel truncated CaV1.2 channel in neonatal rat heart. J. Biol. Chem. 2015, 290, 9262–9272. [Google Scholar] [CrossRef] [PubMed]
- N'Gouemo, P.; Akinfiresoye, L.R.; Allard, J.S.; Lovinger, D.M. Alcohol withdrawal-induced seizure susceptibility is associated with an upregulation of CaV1.3 channels in the rat inferior colliculus. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef] [PubMed]
- Rougier, J.S.; Albesa, M.; Abriel, H.; Viard, P. Neuronal precursor cell-expressed developmentally down-regulated 4-1 (NEDD4-1) controls the sorting of newly synthesized CaV1.2 calcium channels. J. Biol. Chem. 2011, 286, 8829–8838. [Google Scholar] [CrossRef] [PubMed]
- Vijverberg, H.P.; Oortgiesen, M.; Leinders, T.; van Kleef, R.G. Metal interactions with voltage- and receptor-activated ion channels. Environ. Health Perspect. 1994, 102 (Suppl. S3), 153–158. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.D.; Reuveny, E.; Narahashi, T. The effect of polyamines on voltage-activated calcium channels in mouse neuroblastoma cells. J. Physiol. 1993, 462, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Solís-Chagoyán, H.; Calixto, E.; Figueroa, A.; Montaño, L.M.; Berlanga, C.; Rodríguez-Verdugo, M.S.; Romo, F.; Jiménez, M.; Gurrola, C.Z.; Riquelme, A.; et al. Microtubule organization and L-type voltage-activated calcium current in olfactory neuronal cells obtained from patients with schizophrenia and bipolar disorder. Schizophr. Res. 2013, 143, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Rawson, N.E.; Gomez, G. Cell and molecular biology of human olfaction. Microsc. Res. Technol. 2002, 58, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Gómez, G.; Rawson, N.E.; Hahn, C.G.; Michaels, R.; Restrepo, D. Characteristics of odorant elicited calcium changes in cultured human olfactory neurons. J. Neurosci. Res. 2000, 62, 737–749. [Google Scholar] [CrossRef]
- Keller, A.; Margolis, F.L. Isolation and characterization of rat olfactory marker protein. J. Biol. Chem. 1976, 251, 6232–6237. [Google Scholar] [PubMed]
- Hartman, B.K.; Margolis, F.L. Immunofluorescence localization of the olfactory marker protein. Brain Res. 1975, 96, 176–180. [Google Scholar] [CrossRef]
- Tohyama, T.; Lee, V.M.; Rorke, L.B.; Marvin, M.; McKay, R.D.; Trojanowski, J.Q. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab. Investig. 1992, 66, 303–313. [Google Scholar] [PubMed]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D. CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Féron, F.; Perry, C.; Girard, S.D.; Mackay-Sim, A. Isolation of adult stem cells from the human olfactory mucosa. Methods Mol. Biol. 2013, 1059, 107–114. [Google Scholar] [PubMed]
- Othman, M.; Lu, C.; Klueber, K.; Winstead, W.; Roisen, F. Clonal analysis of adult human olfactory neurosphere forming cells. Biotechnol. Histochem. 2005, 80, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Park, J.S.; Jeong, H.S. Neural differentiation of human adipose tissue-derived stem cells involves activation of the Wnt5a/JNK signalling. Stem Cells Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.M.; Cheng, B.; Beyer, A.I.; Mulvaney, U.S.; Wilkinson, M.B.; Fomin, M.E.; Muench, M.O. A quantitative assessment of the content of hematopoietic stem cells in mouse and human endosteal-bone marrow: A simple and rapid method for the isolation of mouse central bone marrow. BMC Hematol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Prè, D.; Nestor, M.W.; Sproul, A.A.; Jacob, S.; Koppensteiner, P.; Chinchalongporn, V.; Zimmer, M.; Yamamoto, A.; Noggle, S.A.; Arancio, O. A time course analysis of the electrophysiological properties of neurons differentiated from human induced pluripotent stem cells (iPSCs). PLoS ONE 2014, 9, e103418. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M. From neuroepithelial cells to neurons: Changes in the physiological properties of neuroepithelial stem cells. Arch. Biochem. Biophys. 2013, 534, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Buzanska, L.; Domanska-Janik, K.; Salvi, R.J.; Stachowiak, M.K. Voltage-sensitive and ligand-gated channels in differentiating neural stem-like cells derived from the nonhematopoietic fraction of human umbilical cord blood. Stem Cells 2005, 23, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Goldsberry, M.E.; Kim, J.; Freeman, J.H. Developmental changes in hippocampal associative coding. J. Neurosci. 2015, 35, 4238–4247. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.G.; Han, L.Y.; Rawson, N.E.; Mirza, N.; Borgmann-Winter, K.; Lenox, R.H.; Arnold, S.E. In vivo and in vitro neurogenesis in human olfactory epithelium. J. Comp. Neurol. 2005, 483, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Bonigk, W.; Bradley, J.; Muller, F.; Sesti, F.; Boekhoff, I.; Ronnett, G.V.; Kaupp, U.B.; Frings, S. The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. J Neurosci. 1999, 19, 5332–5347. [Google Scholar] [PubMed]
- Stephan, A.B.; Shum, E.Y.; Hirsh, S.; Cygnar, K.D.; Reisert, J.; Zhao, H. ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc. Natl. Acad. Sci. USA 2009, 106, 11776–11781. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, T.; Kashiwayanagi, M.; Iijima, T.; Murakami, M. Involvement of the calcium channel β3 subunit in olfactory signal transduction. Biochem. Biophys. Res. Commun. 2007, 355, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.H.; Otsuguro, K.; Ito, S.; Saito, T.; Habara, Y. T-type Ca2+ channels contribute to IBMX/forskolin- and K+-induced Ca2+ transients in porcine olfactory receptor neurons. Neurosci. Res. 2007, 7, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Otsuguro, K.; Gautam, S.H.; Ito, S.; Habara, Y.; Saito, T. Characterization of forskolin-induced Ca2+ signals in rat olfactory receptor neurons. J. Pharmacol. Sci. 2005, 97, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Shibata, S.; Fukuda, K.; Sato, M.; Satoh, E.; Nagoshi, N.; Minematsu, T.; Matsuzaki, Y.; Akazawa, C.; Toyama, Y.; et al. The dual origin of the peripheral olfactory system: Placode and neural crest. Mol. Brain 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Nickell, M.D.; Breheny, P.; Stromberg, A.J.; McClintock, T.S. Genomics of mature and immature olfactory sensory neurons. J. Comp. Neurol. 2012, 520, 2608–2629. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, M.; Piacentini, R.; Casalbore, P.; Budoni, M.; Pallini, R.; Azzena, G.B.; Grassi, C. Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. Eur. J. Neurosci. 2006, 23, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Benítez-King, G.; Valdés-Tovar, M.; Trueta, C.; Galván-Arrieta, T.; Argueta, J.; Alarcón, S.; Lora-Castellanos, A.; Solís-Chagoyán, H. The microtubular cytoskeleton of olfactory neurons derived from patients with schizophrenia or with bipolar disorder: Implications for biomarker characterization, neuronal physiology and pharmacological screening. Mol. Cell. Neurosci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yunker, A.M.; Sharp, A.H.; Sundarraj, S.; Ranganathan, V.; Copeland, T.D.; McEnery, M.W. Immunological characterization of T-type voltage-dependent calcium channel CaV3.1 (α1G) and CaV3.3 (α1I) isoforms reveal differences in their localization, expression, and neural development. Neuroscience 2003, 117, 321–335. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Op De Beeck, L.; Verlooy, J.E.; van Buul-Offers, S.C.; Du Caju, M.V. Detection of serum insulin-like growth factor binding proteins on western ligand blots by biotinylated IGF and enhanced chemiluminescence. J. Endocrinol. 1997, 154, R1–R5. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [PubMed]
- Kajita, J.; Yamaguchi, H. Calcium mobilization by muscarinic cholinergic stimulation in bovine single airway smooth muscle. Am. J. Physiol. 1993, 264, L496–L503. [Google Scholar] [PubMed]
- Carbajal, V.; Vargas, M.H.; Flores-Soto, E.; Martinez-Cordero, E.; Bazan-Perkins, B.; Montaño, L.M. LTD4 induces hyperresponsiveness to histamine in bovine airway smooth muscle: Role of SR-ATPase Ca2+ pump and tyrosine kinase. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 288, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Perusquía, M.; Flores-Soto, E.; Sommer, B.; Campuzano-González, E.; Martínez-Villa, I.; Martínez-Banderas, A.I.; Montaño, L.M. Testosterone-induced relaxation involves L-type and store-operated Ca2+ channels blockade, and PGE 2 in guinea pig airway smooth muscle. Pflügers Arch. 2015, 467, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef] [PubMed]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solís-Chagoyán, H.; Flores-Soto, E.; Reyes-García, J.; Valdés-Tovar, M.; Calixto, E.; Montaño, L.M.; Benítez-King, G. Voltage-Activated Calcium Channels as Functional Markers of Mature Neurons in Human Olfactory Neuroepithelial Cells: Implications for the Study of Neurodevelopment in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2016, 17, 941. https://doi.org/10.3390/ijms17060941
Solís-Chagoyán H, Flores-Soto E, Reyes-García J, Valdés-Tovar M, Calixto E, Montaño LM, Benítez-King G. Voltage-Activated Calcium Channels as Functional Markers of Mature Neurons in Human Olfactory Neuroepithelial Cells: Implications for the Study of Neurodevelopment in Neuropsychiatric Disorders. International Journal of Molecular Sciences. 2016; 17(6):941. https://doi.org/10.3390/ijms17060941
Chicago/Turabian StyleSolís-Chagoyán, Héctor, Edgar Flores-Soto, Jorge Reyes-García, Marcela Valdés-Tovar, Eduardo Calixto, Luis M. Montaño, and Gloria Benítez-King. 2016. "Voltage-Activated Calcium Channels as Functional Markers of Mature Neurons in Human Olfactory Neuroepithelial Cells: Implications for the Study of Neurodevelopment in Neuropsychiatric Disorders" International Journal of Molecular Sciences 17, no. 6: 941. https://doi.org/10.3390/ijms17060941
APA StyleSolís-Chagoyán, H., Flores-Soto, E., Reyes-García, J., Valdés-Tovar, M., Calixto, E., Montaño, L. M., & Benítez-King, G. (2016). Voltage-Activated Calcium Channels as Functional Markers of Mature Neurons in Human Olfactory Neuroepithelial Cells: Implications for the Study of Neurodevelopment in Neuropsychiatric Disorders. International Journal of Molecular Sciences, 17(6), 941. https://doi.org/10.3390/ijms17060941