Regulation of Intestinal Epithelial Calcium Transport Proteins by Stanniocalcin-1 in Caco2 Cells
Abstract
:1. Introduction
2. Results
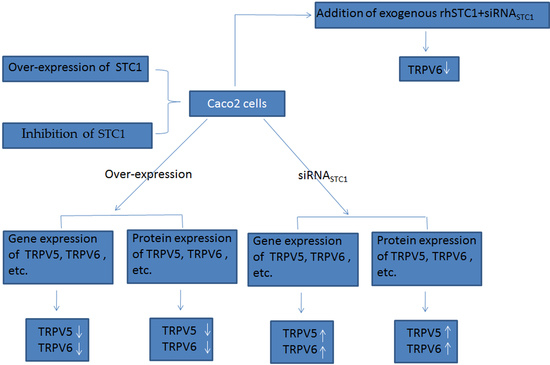
2.1. Expression of STC1 in Transfected Caco2 Cells
2.2. Effect of STC1 on the Expression of Calcium-Transporting Proteins
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Construction of STC1 Expression Vector
4.3. Design and Synthesis of siRNA
4.4. Transfection and Treatments of Caco2 Cells
4.5. Real-Time PCR
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoenderop, J.G.; Nilius, B.; Bindels, R.J. Calcium absorption across epithelia. Physiol. Rev. 2005, 85, 373–422. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Nemere, I. Regulation of intestinal calcium transport. Annu. Rev. Nutr. 2008, 28, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-H.; Choi, K.-C.; Oh, G.T.; Jeung, E.-B. Effect of dietary calcium and 1,25-(OH)2D3 on the expression of calcium transport genes in calbindin-D9k and-D28k double knockout mice. Biochem. Biophys. Res. Commun. 2009, 379, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; An, B.; Yang, H.; Jeung, E. Regulation and molecular mechanisms of calcium transport genes: Do they play a role in calcium transport in the uterine endometrium. J. Physiol. Pharmacol. 2011, 62, 499–504. [Google Scholar] [PubMed]
- Johnson, J.A.; Grande, J.P.; Roche, P.C.; Sweeney, W.; Avner, E.; Kumar, R. 1 alpha, 25-dihydroxyvitamin D3 receptor ontogenesis in fetal renal development. Am. J. Physiol. Ren. Physiol. 1995, 269, F419–F428. [Google Scholar]
- Den Dekker, E.; Hoenderop, J.G.; Nilius, B.; Bindels, R.J. The epithelial calcium channels, TRPV5 & TRPV6: From identification towards regulation. Cell Calcium 2003, 33, 497–507. [Google Scholar] [PubMed]
- Kip, S.N.; Gray, N.W.; Burette, A.; Canbay, A.; Weinberg, R.J.; Strehler, E.E. Changes in the expression of plasma membrane calcium extrusion systems during the maturation of hippocampal neurons. Hippocampus 2006, 16, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Rourke, K.; Coe, S.; Kohn, C.; Rosol, T.; Mendoza, F.; Toribio, R. Cloning, comparative sequence analysis and mRNA expression of calcium-transporting genes in horses. Gen. Comp. Endocrinol. 2010, 167, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.F.; Dimattia, G.E. The stanniocalcin family of proteins. J. Exp. Zool. A Ecol. Genet. Physiol. 2006, 305, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Yang, S.J.; Wu, L.M.; Xi, Z.-F.; Chen, S.; Dong, S.-Q.; Wang, J.-L.; Guo, D.-Z. Expression and localization of Stanniocalcin 1 in bovine osteoblasts. Pak. Vet. J. 2012, 32, 242–246. [Google Scholar]
- Yoshiko, Y.; Aubin, J.E. Stanniocalcin 1 as a pleiotropic factor in mammals. Peptides 2004, 25, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, G.; Delbecchi, L.; Loiselle, M.C.; Ster, C.; Wagner, G.F.; Talbot, B.G.; Lacasse, P. Serum levels of stanniocalcin-1 in Holstein heifers and cows. Domest. Anim. Endocrinol. 2009, 36, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.S.; Cepeda, M.A.; Zhang, Q.Q.; Rosen, C.A.; Vozzolo, B.L. Human stanniocalcin: A possible hormonal regulator of mineral metabolism. Proc. Natl. Acad. Sci. USA 1996, 93, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Yeung, B.; Law, A.; Wong, C.K. Evolution and roles of stanniocalcin. Mol. Cell. Endocrinol. 2012, 349, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.F.; de Niu, P.; Jaworski, E.; Radman, D.; Chiarot, C. Development of a dose-response bioassay for stanniocalcin in fish. Mol. Cell. Endocrinol. 1997, 128, 19–28. [Google Scholar] [CrossRef]
- De Niu, P.; Radman, D.P.; Jaworski, E.M.; Deol, H.; Gentz, R.; Su, J.; Olsen, H.S.; Wagner, G.F. Development of a human stanniocalcin radioimmunoassay: Serum and tissue hormone levels and pharmacokinetics in the rat. Mol. Cell. Endocrinol. 2000, 162, 131–144. [Google Scholar] [CrossRef]
- Madsen, K.L.; Tavernini, M.M.; Yachimec, C.; Mendrick, D.L.; Alfonso, P.J.; Buergin, M.; Olsen, H.S.; Antonaccio, M.J.; Thomson, A.B.R.; Fedorak, R.N. Stanniocalcin: A novel protein regulating calcium and phosphate transport across mammalian intestine. Am. J. Physiol. 1998, 274, G96–G102. [Google Scholar] [PubMed]
- Wagner, G.F.; Vozzolo, B.L.; Jaworski, E.; Haddad, M.; Kline, R.L.; Olsen, H.S.; Rosen, C.A.; Davidson, M.B.; Renfro, J.L. Human stanniocalcin inhibits renal phosphate excretion in the rat. J. Bone Miner. Res. 1997, 12, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Chantret, I.; Barbat, A.; Dussaulx, E.; Brattain, M.G.; Zweibaum, A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: A survey of twenty cell lines. Cancer Res. 1988, 48, 1936–1942. [Google Scholar] [PubMed]
- Tseng, D.Y.; Chou, M.Y.; Tseng, Y.C.; Hsiao, C.-D.; Huang, C.-J.; Kaneko, T.; Hwang, P.-P. Effects of stanniocalcin 1 on calcium uptake in zebrafish (Danio rerio) embryo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R549–R557. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Hamad, D.; Bick, R.; Wu, G.Y.; Christensen, B.M.; Razeghi, P.; Poindexter, B.; Taegtmeyer, H.; Wamsley, A.; Padda, R.; Entman, M. Stanniocalcin-1 is a naturally occurring L-channel inhibitor in cardiomyocytes: Relevance to human heart failure. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H442–H448. [Google Scholar] [CrossRef] [PubMed]
- Verbost, P.M.; Flik, G.; Fenwick, J.C.; Greco, A.-M.; Pang, P.K.T.; Bonga, S.E.W. Branchial calcium uptake: Possible mechanisms of control by stanniocalcin. Fish Physiol. Biochem. 1993, 11, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Bindels, R.; Ramakers, P.; Dempster, J.; Hartog, A.; van Os, C. Role of Na+/Ca2+ exchange in transcellular Ca2+ transport across primary cultures of rabbit kidney collecting system. Pflügers Arch. 1992, 420, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.T.; Yamamoto, H.; Takei, Y.; Masuda, M.; Otani, A.; Kozai, M.; Ikeda, S.; Nakahashi, O.; Tanaka, S.; Taketani, Y.; et al. Up-regulation of stanniocalcin 1 expression by 1,25-dihydroxy vitamin D3 and parathyroid hormone in renal proximal tubular cells. J. Clin. Biochem. Nutr. 2012, 50, 227–233. [Google Scholar] [CrossRef] [PubMed]



| Gene | Genebank Accession No. | Primer (5′–3′) | Product Length (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| TRPV6 | NM_018646 | GGACAACACCCTCTTACAGCA(sense) | 224 | 60 |
| CCAGCACCATGAAGGCATA(anti-sense) | ||||
| TRPV5 | NM_019841.4 | TCTTAGGCAACTTCTACTGGACTG(sense) | 223 | 60 |
| ACGCACCAGGTTCACATTCT(anti-sense) | ||||
| PMCA1 | NM_001001323 | CAGCAGGAGAACCAGAACCA(sense) | 159 | 60 |
| CAGTGACCATCCGCACAGTAA(anti-sense) | ||||
| NCX1 | XM_005264514.1 | TGTGCATCTCAGCAATGTCA(sense) | 230 | 60 |
| TTCCTCGAGCTCCAGATGTT(anti-sense) | ||||
| VDR | NM_000376 | GTGGACATCGGCATGATGAAG(sense) | 181 | 60 |
| GGTCGTAGGTCTTATGGTGGG(anti-sense) | ||||
| GAPDH | NM_002046 | TGCACCACCAACTGCTTAGC(sense) | 87 | 60 |
| GGCATGGACTGTGGTCATGAG(anti-sense) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, J.; Guo, R.; Wan, C.; Wu, L.; Yang, S.; Guo, D. Regulation of Intestinal Epithelial Calcium Transport Proteins by Stanniocalcin-1 in Caco2 Cells. Int. J. Mol. Sci. 2016, 17, 1095. https://doi.org/10.3390/ijms17071095
Xiang J, Guo R, Wan C, Wu L, Yang S, Guo D. Regulation of Intestinal Epithelial Calcium Transport Proteins by Stanniocalcin-1 in Caco2 Cells. International Journal of Molecular Sciences. 2016; 17(7):1095. https://doi.org/10.3390/ijms17071095
Chicago/Turabian StyleXiang, Jinmei, Rui Guo, Chunyun Wan, Liming Wu, Shijin Yang, and Dingzong Guo. 2016. "Regulation of Intestinal Epithelial Calcium Transport Proteins by Stanniocalcin-1 in Caco2 Cells" International Journal of Molecular Sciences 17, no. 7: 1095. https://doi.org/10.3390/ijms17071095
APA StyleXiang, J., Guo, R., Wan, C., Wu, L., Yang, S., & Guo, D. (2016). Regulation of Intestinal Epithelial Calcium Transport Proteins by Stanniocalcin-1 in Caco2 Cells. International Journal of Molecular Sciences, 17(7), 1095. https://doi.org/10.3390/ijms17071095