A Homogeneous Polysaccharide from Fructus Schisandra chinensis (Turz.) Baill Induces Mitochondrial Apoptosis through the Hsp90/AKT Signalling Pathway in HepG2 Cells
Abstract
:1. Introduction
2. Results
2.1. Extraction, Separation and Purification of S. chinensis Polysaccharide-0-1
2.2. Antiproliferative Activity of S. chinensis Polysaccharide-0-1
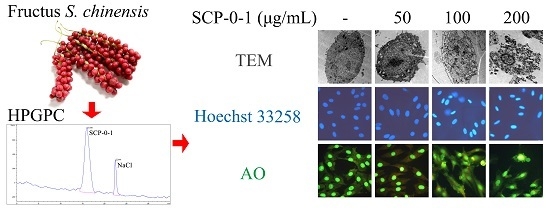
2.3. Cell Morphology Demonstrated that S. chinensis Polysaccharide-0-1 Induced Apoptosis Rather than Autophagy
2.4. Changes in Proteins Expression Involved in Apoptosis and Autophagy
2.5. Changes in Protein Expression Involved in Mitogen-Activated Protein Kinase/Phosphoinositide 3-Kinase/Heat Shock Protein 90 Signalling Pathways
2.6. Inhibition by S. chinensis Polysaccharide-0-1 Is Attributed to the Repression of the Heat Shock Protein 90/Protein Kinase B Signalling Pathway
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction, Separation and Purification of S. chinensis Polysaccharide-0-1
4.3. Homogeneity and Molecular Weight
4.4. Cell Culture
4.5. 3-[4,5-Dimethyl-2-thiazolyl]-2,5-diphenyltetrazolium bromide (MTT) Assay
4.6. Flow Cytometric Cell-Cycle Analysis
4.7. Annexin-V/Phosphatidylinositol Staining
4.8. Phase Contrast Microscopy
4.9. Transmission Electron Microscopy
4.10. Hoechst 33258 Staining
4.11. Detection of Acidic Vesicular Organelles
4.12. Western Blotting
4.13. Cytochrome C Release
4.14. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA-Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Zhang, S.W. Liver cancer epidemic in China: Past, present and future. Semin. Cancer Biol. 2011, 21, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Zheng, R.S.; Baade, P.D.; Zhang, S.W.; Zeng, H.M.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA-Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Margină, D.; Ilie, M.; Grădinaru, D.; Androutsopoulos, V.P.; Kouretas, D.; Tsatsakis, A.M. Natural products—Friends or foes? Toxicol. Lett. 2015, 236, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhang, H.Y. Western-medicine-validated anti-tumor agents and traditional Chinese medicine. Trends Mol. Med. 2008, 14, 1–2. [Google Scholar] [CrossRef] [PubMed]
- National Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China; Chemical Industry Press: Beijing, China, 2015; Part 1; pp. 66–67. [Google Scholar]
- Jiang, Y.; Zhang, Q.; Bao, J.; Du, C.; Wang, J.; Tong, Q.; Liu, C. Schisandrin B suppresses glioma cell metastasis mediated by inhibition of mTOR/MMP-9 signal pathway. Biomed. Pharmacother. 2015, 74, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Venkanna, A.; Siva, B.; Poornima, B.; Vadaparthi, P.R.; Prasad, K.R.; Reddy, K.A.; Reddy, G.B.; Babu, K.S. Phytochemical investigation of sesquiterpenes from the fruits of Schisandra chinensis and their cytotoxic activity. Fitoterapia 2014, 95, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.J.; Choi, I.W.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Induction of reactive oxygen species–mediated apoptosis by purified Schisandrae semen essential oil in human leukemia U937 cells through activation of the caspase cascades and nuclear relocation of mitochondrial apoptogenic factors. Nutr. Res. 2015, 35, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Casarin, E.; Dall’Acqua, S.; Smejkal, K.; Slapetová, T.; Innocenti, G.; Carrara, M. Molecular mechanisms of antiproliferative effects induced by Schisandra-derived dibenzocyclooctadiene lignans (+)-deoxyschisandrin and (−)-gomisin N in human tumour cell lines. Fitoterapia 2014, 98, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Lee, K.M.; Yoo, J.H.; Lee, H.J.; Kim, C.Y.; Nho, C.W. Dibenzocyclooctadiene lignans, gomisins J and N inhibit the Wnt/β-catenin signaling pathway in HCT116 cells. Biochem. Biophys. Res. Commun. 2012, 428, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Gnabre, J.; Unlu, I.; Chang, T.C.; Lisseck, P.; Bourne, B.; Scolnik, R.; Jacobsen, N.E.; Bates, R.; Huang, R.C. Isolation of lignans from Schisandra chinensis with anti-proliferative activity in human colorectal carcinoma: Structure-activity relationships. J. Chromatogr. B 2010, 878, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.Y.; Jang, J.Y.; Choi, Y.H.; Choi, Y.W.; Choi, B.T. Inhibitory effects of 1-O-methyl-fructofuranose from Schisandra chinensis fruit on melanogenesis in B16F0 melanoma cells. J. Ethnopharmacol. 2010, 132, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Biao, Z.; Zhen, L.; Xun, H. Inhibiting cancer metastasis via targeting NAPDH oxidase 4. Biochem. Pharmacol. 2013, 86, 253–266. [Google Scholar]
- Li, W.L.; Xin, H.W.; Su, M.W.; Xiong, L. Inhibitory effects of schisandrin A and schisandrin B on CYP3A activity. Methods Find Exp. Clin. Pharmacol. 2010, 32, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, Y.W.; Park, C.; Jin, C.Y.; Lee, Y.J.; Park da, J.; Kim, S.G.; Kim, G.Y.; Choi, I.W.; Hwang, W.D.; et al. Apoptosis induction of human leukemia U937 cells by gomisin N, a dibenzocyclooctadiene lignan, isolated from Schizandra chinensis Baill. Food Chem. Toxicol. 2010, 48, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ying, Z.; Zu, Y.; Fu, Y.; Wang, W. Composition and biological activities of the essential oil from Schisandra chinensis obtained by solvent-free microwave extraction. Lebenson. Wiss. Technol. 2011, 44, 2047–2052. [Google Scholar] [CrossRef]
- Liu, S.J.; Qu, H.M.; Ren, Y.P. SCP, a polysaccharide from Schisandra chinensis, induces apoptosis in human renal cell carcinoma Caki-1 cells through mitochondrial-dependent pathway via inhibition of ERK activation. Tumor Biol. 2014, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.M.; Liu, S.J.; Zhang, C.Y. Antitumor and antiangiogenic activity of Schisandra chinensis polysaccharide in a renal cell carcinoma model. Int. J. Biol. Macromol. 2014, 66, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Mao, G.; Zhang, M.; Zou, Y.; Feng, W.; Gu, X.; Zhu, Y.; Mao, R.; Yang, L.; Wu, X. Enhanced antitumor and reduced toxicity effect of Schisanreae polysaccharide in 5-Fu treated Heps-bearing mice. Int. J. Biol. Macromol. 2014, 63, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Mao, G.; Zhang, M.; Zou, Y.; Feng, W.; Gu, X.; Zhu, Y.; Mao, R.; Yang, L.; Wu, X. Antitumor and immunomodulatory activity of a water-soluble low molecular weight polysaccharide from Schisandra chinensis (Turcz.) Baill. Food Chem. Toxicol. 2013, 55, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, X.; Yang, Z.; Zhao, X. Honokiol induces caspase-independent paraptosis via reactive oxygen species production that is accompanied by apoptosis in leukemia cells. Biochem. Biophys. Res. Commun. 2013, 3, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Miyazawa, K.; Naito, M.; Toyotake, J.; Tauchi, T.; Itoh, M.; You, A.; Hayashi, Y.; Georgescu, M.M.; Kondo, Y.; et al. Vitamin K2 induces autophagy and apoptosis simultaneously in leukemia cells. Autophagy 2008, 4, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Jänicke, R.U.; Sprengart, M.L.; Wati, M.R.; Porter, A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef] [PubMed]
- Uren, A.G.; Wong, L.; Pakusch, M.; Fowler, K.J.; Burrows, F.J.; Vaux, D.L.; Choo, K.H. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr. Biol. 2000, 10, 1319–1328. [Google Scholar] [CrossRef]
- Wu, J.; Dang, Y.; Su, W.; Liu, C.; Ma, H.; Shan, Y.; Pei, Y.; Wan, B.; Guo, J.; Yu, L. Molecular cloning and characterization of rat LC3A and LC3B—Two novel markers of autophagosome. Biochem. Biophys. Res. Commun. 2006, 339, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers, G.T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.W.; Ho, Y.S.; Wang, Y.J. Arsenic trioxide induces autophagy and apoptosis in human glioma cells in vitro and in vivo through downregulation of survivin. J. Mol. Med. 2011, 89, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; McConkey, D.J.; Hong, D.S.; Kurzrock, R. Autophagy as a target for anticancer therapy. Nat. Rev. Clin. Oncol. 2011, 8, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Alison, M.; Paul, W. HSP90 as a new therapeutic target for cancer therapy: The story unfolds. Expert Opin. Biol. Ther. 2002, 2, 3–24. [Google Scholar]
- Zhao, L.M.; Jia, Y.L.; Ma, M.; Duan, Y.Q.; Liu, L.H. Prevention effects of Schisandra polysaccharide on radiation-induced immune system dysfunction. Int. J. Biol. Macromol. 2015, 76, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Bacus, S.S.; Gudkov, A.V.; Lowe, M.; Lyass, L.; Yung, Y.; Komarov, A.P.; Keyomarsi, K.; Yarden, Y.; Seger, R. Taxol-induced apoptosis depends on MAP kinase pathways (ERK and p38) and is independent of p53. Oncogene 2001, 20, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ko, L.J.; Jayaraman, L. Prives C: P53 Levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996, 10, 2438–2451. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Sheng, Y.; Kotsuji, F.; Tsang, B.K. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 2000, 60, 5659–5666. [Google Scholar] [PubMed]
- Mishra, O.P.; Randis, T.; Ashraf, Q.M. Delivoria-Papadopoulos M: Hypoxia-induced Bax and Bcl-2 protein expression, caspase-9 activation, DNA fragmentation, and lipid peroxidation in mitochondria of the cerebral cortex of newborn piglets: The role of nitric oxide. Neuroscience 2006, 141, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.M.; Greengrass, P.M.; Cavalla, D. The role of mitochondria in apoptosis. Drug News Perspect. 2000, 13, 378–384. [Google Scholar] [PubMed]
- Ogbourne, S.M.; Suhrbier, A.; Jones, B.; Cozzi, S.J.; Boyle, G.M.; Morris, M.; McAlpine, D.; Johns, J.; Scott, T.M.; Sutherland, K.P.; et al. Antitumor activity of 3-ingenyl angelate: Plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004, 64, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Schweizer, M.; Cossarizza, A.; Franceschi, C. Control of apoptosis by the cellular ATP level. FEBS Lett. 1996, 378, 107–110. [Google Scholar] [CrossRef]
- Bayir, H.; Fadeel, B.; Palladino, M.J.; Witasp, E.; Kurnikov, I.V.; Tyurina, Y.Y.; Tyurin, V.A.; Amoscato, A.A.; Jiang, J.; Kochanek, P.M.; et al. Apoptotic interactions of cytochrome c: Redox flirting with anionic phospholipids within and outside of mitochondria. Biochim. Biophys. Acta 2006, 1757, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, T.; Krajewski, S.; Krajewska, M.; Wang, H.G.; Lin, H.K.; Liebermann, D.A.; Hoffman, B.; Reed, J.C. Tumor suppressor p53 is a regulator of Bcl-2 and Bax gene expression in vitro and in vivo. Oncogene 1994, 9, 1799–1805. [Google Scholar] [PubMed]
- Taylor, W.R.; Stark, G.R. Regulation of the G2/M transition by p53. Oncogene 2001, 20, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Tolkovsky, A.M. Necrosis, apoptosis, and autophagy: Mechanisms of neuronal and glial cell death. Neuromethods 2011, 56, 305–330. [Google Scholar]
- Altman, B.J.; Rathmell, J.C. Metabolic stress in autophagy and cell death pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a008763. [Google Scholar] [CrossRef] [PubMed]
- Annick, N.; Lionel, L.; Carine, M. Autophagy as a mediator of chemotherapy-induced cell death in cancer. Biochem. Pharmacol. 2011, 82, 427–434. [Google Scholar]
- Levy, J.M.M.; Thorburn, A. Targeting autophagy during cancer therapy to improve clinical outcomes. Pharmacol. Ther. 2011, 131, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.M.; Ding, W.X.; Gao, W. Autophagy in the liver. Hepatology 2008, 47, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Qi, Y.; Wadham, C.; Wang, L.; Warren, A.; Di, W.; Xia, P. FTY720 induces necrotic cell death and autophagy in ovarian cancer cells: A protective role of autophagy. Autophagy 2010, 6, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Wallington-Beddoe, C.T.; Hewson, J.; Bradstock, K.F.; Bendall, L.J. FTY720 produces caspase-independent cell death of acute lymphoblastic leukemia cells. Autophagy 2011, 7, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Sui, X.; Yao, J.; Xie, J.; Jiang, L.; Zhou, Y.; Pan, H.; Han, W. SKF-96365 activates cytoprotective autophagy to delay apoptosis in colorectal cancer cells through inhibition of the calcium/CaMKIIγ/AKT-mediated pathway. Cancer Lett. 2016, 372, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhou, J.; Shu, G.; Liu, D.C.; Zhou, J.; Chen, J.; Yuan, L. Restoration of klotho gene expression induces apoptosis and autophagy in gastric cancer cells: Tumor suppressive role of klotho in gastric cancer. Cancer Cell. Int. 2013, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Xie, B.; Ren, F.; Liu, D.C.; Zhou, J.; Li, Q.; Chen, J.; Yuan, L.; Zhou, J. Restoration of klotho expression induces apoptosis and autophagy in hepatocellular carcinoma cells. Cell Oncol. 2013, 36, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Zes, M.G.; Sipos, F. Anti-tumor immunity, autophagy and chemotherapy. World J. Gastroenterol. 2012, 18, 6537–6540. [Google Scholar]
- Li, T.; Tang, Z.H.; Xu, W.S.; Wu, G.S.; Wang, Y.F.; Chang, L.L.; Zhu, H.; Chen, X.P.; Wang, Y.T.; Chen, Y.; et al. Platycodin D triggers autophagy through activation of extracellular signal-regulated kinase in hepatocellular carcinoma HepG2 cells. Eur. J. Pharmacol. 2015, 749, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Khuri, F.R. Targeting the PI3K/Akt/mTOR Pathway. Infect. Agents Dis. 2009, 4, 395–401. [Google Scholar]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, T.M.; Leal, J.F.; Seger, R.; Taya, Y.; Oren, M. Cross-talk between Akt, p53 and Mdm2: Possible implications for the regulation of apoptosis. Oncogene 2002, 21, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Tamatani, M.; Mitsuda, N.; Namikawa, K.; Kiyama, H.; Miyake, S.; Tohyama, M. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J. Neurochem. 1999, 73, 2037–2046. [Google Scholar] [PubMed]
- Xia, Z.; Dickens, M.; Raingeaud, J.; Davis, R.J.; Greenberg, M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995, 270, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L. Heat shock protein 90: The cancer chaperone. J. Biosci. 2007, 32, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L.; Neckers, K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutics—An update. Expert Opin. Emerg. Drugs 2005, 10, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Lin, Z.; Zhao, B.; Wang, Y.; Peng, R.; Wang, M.; Lu, C.; Shi, G.; Shen, Y. 17-DMCHAG, a new geldanamycin derivative, inhibits prostate cancer cells through Hsp90 inhibition and survivin downregulation. Cancer Lett. 2015, 362, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, K.; Ailer, G.; Vlotides, G.; Spoettl, G.; Maurer, J.; Göke, B.; Beuschlein, F.; Auernhammer, C.J. Potent antitumor activity of the novel HSP90 inhibitors AUY922 and HSP990 in neuroendocrine carcinoid cells. Int. J. Oncol. 2013, 43, 1824–1832. [Google Scholar] [PubMed]
- Wang, H.; Wang, H.; Shi, S.; Duan, J.; Wang, S. Structural characterization of a homogalacturonan from Capparis spinosa L. fruits and anti-complement activity of its sulfated derivative. Glycoconj. J. 2012, 29, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Nencioni, A.; Wille, L.; Dal, B.G.; Boy, D.; Cirmena, G.; Wesselborg, S.; Belka, C.; Brossart, P.; Patrone, F.; Ballestrero, A. Cooperative cytotoxicity of proteasome inhibitors and tumor necrosis factor-related apoptosis-inducing ligand in chemoresistant Bcl-2-overexpressing cells. Clin. Cancer Res. 2005, 11, 4259–4265. [Google Scholar] [CrossRef] [PubMed]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Shi, S.; Wang, H.; Li, N.; Su, J.; Chou, G.; Wang, S. A Homogeneous Polysaccharide from Fructus Schisandra chinensis (Turz.) Baill Induces Mitochondrial Apoptosis through the Hsp90/AKT Signalling Pathway in HepG2 Cells. Int. J. Mol. Sci. 2016, 17, 1015. https://doi.org/10.3390/ijms17071015
Chen Y, Shi S, Wang H, Li N, Su J, Chou G, Wang S. A Homogeneous Polysaccharide from Fructus Schisandra chinensis (Turz.) Baill Induces Mitochondrial Apoptosis through the Hsp90/AKT Signalling Pathway in HepG2 Cells. International Journal of Molecular Sciences. 2016; 17(7):1015. https://doi.org/10.3390/ijms17071015
Chicago/Turabian StyleChen, Yonglin, Songshan Shi, Huijun Wang, Ning Li, Juan Su, Guixin Chou, and Shunchun Wang. 2016. "A Homogeneous Polysaccharide from Fructus Schisandra chinensis (Turz.) Baill Induces Mitochondrial Apoptosis through the Hsp90/AKT Signalling Pathway in HepG2 Cells" International Journal of Molecular Sciences 17, no. 7: 1015. https://doi.org/10.3390/ijms17071015
APA StyleChen, Y., Shi, S., Wang, H., Li, N., Su, J., Chou, G., & Wang, S. (2016). A Homogeneous Polysaccharide from Fructus Schisandra chinensis (Turz.) Baill Induces Mitochondrial Apoptosis through the Hsp90/AKT Signalling Pathway in HepG2 Cells. International Journal of Molecular Sciences, 17(7), 1015. https://doi.org/10.3390/ijms17071015