Inhibition of AQP1 Hampers Osteosarcoma and Hepatocellular Carcinoma Progression Mediated by Bone Marrow-Derived Mesenchymal Stem Cells
Abstract
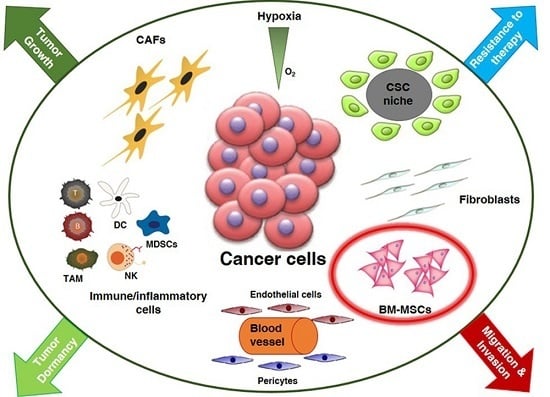
:1. Introduction
2. Results
2.1. Enhancement of Aquaporin 1 (AQP1) Levels in Osteosarcoma (OS) and Hepatocellular Carcinoma (HCC) Cells by Conditioned Medium from Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs)
2.2. Inhibition of AQP1 Hampers OS and HCC Cell Migration Bone Marrow-Derived Mesenchymal Stem Cells-Conditioned Medium (BM-MSC-CM)-Mediated
2.3. Inhibition of AQP1 Hampers BM-MSCs-CM-Dependent OS and HCC Cell Invasion
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Bone Marrow Mesenchymal Stem Cell Isolation and Characterization (BM-MSCs)
4.3. Western Blot Analysis
4.4. Wound Healing Assay
4.5. Cell Invasion Assay
4.6. Statistics
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| AQPs | aquaporins |
| α-MEM | α-modification of minimal essential medium |
| BM-MSCs | bone marrow-derived mesenchymal stem cells |
| CAFs | carcinoma associated fibroblasts |
| CM | conditioned medium |
| CSCs | cancer stem cells |
| DC | dendritic cell |
| DMEM | Dulbecco’s modified Eagle medium |
| ECL | enhanced chemiluminiscence |
| EMT | epithelial-to-mesenchymal transition |
| FBS | fetal bovine serum |
| FCS | fetal calf serum |
| HCC | hepatocellular carcinoma |
| MDSCs | myeloid-derived-suppressor cells |
| NK | natural killer |
| OS | osteosarcoma |
| PBS | phosphate buffered saline |
| SD | standard deviation |
| TAM | tumor associated macrophages |
| TEA | tetraethylammonium chloride |
| TME | tumor microenvironment |
References
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.M.; Fusenig, N.E. Friends or foes—Bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 2004, 4, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Khakoo, A.Y.; Pati, S.; Anderson, S.A.; Reid, W.; Elshal, M.F.; Rovira, I.I.; Nguyen, A.T.; Malide, D.; Combs, C.A.; Hall, G.; et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J. Exp. Med. 2006, 203, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ginestier, C.; Ou, S.J.; Clouthier, S.G.; Patel, S.H.; Monville, F.; Korkaya, H.; Heath, A.; Dutcher, J.; Kleer, C.G.; et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011, 71, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ok Lee, S.; Liang, L.; Huang, C.K.; Li, L.; Wen, S.; Chang, C. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene 2014, 33, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intracellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Shen, Q.; Yang, X.; Qiu, Y.; Zhang, W. The role of extracellular vesicles: An epigenetic view of the cancer microenvironment. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ranieri, G.; Annese, T.; Nico, B. Aquaporins in cancer. Biochim. Biophys. Acta 2014, 1840, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Z.; Song, Y.; Zhang, P.; Hu, J.; Bai, C. Expression of aquaporin 5 increases proliferation and metastasis potential of lung cancer. J. Pathol. 2010, 221, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Chae, Y.K.; Woo, J.; Kim, M.S.; Park, J.C.; Lee, J.; Soria, J.C.; Jang, S.J.; Sidransky, D.; Moon, C. Role of human aquaporin 5 in colorectal carcinogenesis. Am. J. Pathol. 2008, 173, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Khan, M.; Gao, H.; Hao, F.; Sun, M.; Zhong, L.; Lu, C.; Feng, X.; Ma, T. Increased differentiation capacity of bone marrow-derived mesenchymal stem cells in aquaporin-5 deficiency. Stem Cells Dev. 2012, 21, 2495–2507. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Eur. J. Physiol. 2008, 456, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins—New players in cancer biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Nico, B.; Ribatti, D. Aquaporins in tumor growth and angiogenesis. Cancer Lett. 2010, 294, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Verkman, A.S. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006, 20, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, T.; Yang, M.; Li, Z.; Li, Z.; Gao, Y. Prognostic value of combined aquaporin 3 and aquaporin 5 overexpression in hepatocellular carcinoma. BioMed Res. Int. 2013, 2013, 206525. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, S.; Liu, J.; Shi, Y.; Wang, J.; Chen, D.; Luo, L.; Qian, Y.; Huang, X.; Wang, H. RNAi-mediated silencing of AQP1 expression inhibited the proliferation, invasion and tumorigenesis of osteosarcoma cells. Cancer Biol. Ther. 2015, 16, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.L.; Regan, J.W.; Yool, A.J. Inhibition of aquaporin-1 water permeability by tetraethylammonium: Involvement of the loop E pore region. Mol. Pharmacol. 2000, 57, 1021–1026. [Google Scholar] [PubMed]
- Wels, J.; Kaplan, R.N.; Rafii, S.; Lyden, D. Migratory neighbors and distant invaders: Tumor-associated niche cells. Genes Dev. 2008, 22, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, J.K.; Shiozawa, Y.; Wang, J.; Mishra, A.; Joseph, J.; Berry, J.E.; McGee, S.; Lee, E.; Sun, H.; et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun. 2013, 4, 1795. [Google Scholar] [CrossRef] [PubMed]
- Bergfeld, S.A.; de Clerck, Y.A. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev. 2010, 29, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lee, Y.W.; Rui, Y.F.; Cheng, T.Y.; Jiang, X.H.; Li, G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res. Ther. 2013, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Gilkes, D.M.; Wong, C.C.; Kshitiz, L.W.; Zhang, H.; Wei, H.; Takano, N.; Schito, L.; Levchenko, A.; Semenza, G.L. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J. Clin. Investig. 2013, 123, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Du, L.; Fan, Q.M.; Tang, Z.; Tang, T.T. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012, 1, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Dong, L.; Yan, K.; Long, H.; Yang, T.T.; Dong, M.Q.; Zhou, Y.; Fan, Q.Y.; Ma, B.A. CXCR4-mediated osteosarcoma growth and pulmonary metastasis is promoted by mesenchymal stem cells through VEGF. Oncol. Rep. 2013, 30, 1753–1761. [Google Scholar] [PubMed]
- Yu, F.X.; Hu, W.J.; He, B.; Zheng, Y.H.; Zhang, Q.Y.; Chen, L. Bone marrow mesenchymal stem cells promote osteosarcoma cell proliferation and invasion. World J. Surg. Oncol. 2015, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, K.; Kitadai, Y.; Tanaka, M.; Sumida, T.; Kodama, M.; Higashi, Y.; Tanaka, S.; Yasui, W.; Chayama, K. Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int. J. Cancer 2010, 15, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, Z.P.; Zhang, S.S.; Jing, Y.Y.; Bu, X.X.; Wang, C.Y.; Sun, K.; Jiang, G.C.; Zhao, X.; Li, R.; et al. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J. Biol. Chem. 2011, 15, 25007–25015. [Google Scholar] [CrossRef] [PubMed]
- Hogan, N.M.; Joyce, M.R.; Murphy, J.M.; Barry, F.P.; O’Brien, T.; Kerin, M.J.; Dwyer, R.M. Impact of mesenchymal stem cell secreted PAI-1 on colon cancer cell migration and proliferation. Biochem. Biophys. Res. Commun. 2013, 14, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, R.; Pelagalli, A.; Nardelli, A.; D’Alterio, C.; Ieranò, C.; Cerchia, L.; Lucarelli, E.; Scala, S.; Zannetti, A. A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Lett. 2016, 370, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Clapp, C.; Martínez de la Escalera, G. Aquaporin-1: A novel promoter of tumor angiogenesis. Trends Endocrinol. Metab. 2006, 17, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Detmers, F.J.M.; de Groot, B.L.; Muller, E.M.; Hinton, A.; Konings, I.B.M.; Sze, M.; Flitsch, S.L.; Grubmuller, H.; Deen, P.M.T. Quaternary ammonium compounds as water channel blockers. J. Biol. Chem. 2006, 281, 14207–14214. [Google Scholar] [CrossRef] [PubMed]
- Muller, E.M.; Hub, J.S.; Grubmuller, H.; de Groot, B.L. Is TEA an inhibitor for human Aquaporin-1? Eur. J. Physiol. 2008, 456, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kim, J.K.; Verkman, A.S. Comparative efficacy of HgCl2 with candidate aquaporin-1 inhibitors DMSO, gold, TEA+ and acetazolamide. FEBS Lett. 2006, 580, 6679–6684. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Iwata, Y.; Miura, S.; Kawada, K. Reinvestigation of drugs and chemicals as aquaporin-1 inhibitors using pressure-induced hemolysis in human erythrocytes. Biol. Pharm. Bull. 2012, 35, 2088–2091. [Google Scholar] [CrossRef] [PubMed]
- Esteva-Font, C.; Jin, B.J.; Phuan, P.W.; Anderson, M.O.; Verkman, A.S. Experimental evaluation of proposed small molecule inhibitors of water channel aquaporin-1. Mol. Pharmacol. 2016, 89, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Dorward, H.S.; Du, A.; Bruhn, M.A.; Wrin, J.; Pei, J.V.; Evdokiou, A.; Price, T.J.; Yool, A.J.; Hardingham, J.E. Pharmacological blockade of aquaporin-1 water channel by AqB013 restricts migration and invasiveness of colon cancer cells and prevents endothelial tube formation in vitro. J. Exp. Clin. Cancer Res. 2016, 35, 36. [Google Scholar] [CrossRef] [PubMed]
- Pierini, M.; Dozza, B.; Lucarelli, E.; Tazzari, P.L.; Ricci, F.; Remondini, D.; di Bella, C.; Giannini, S.; Donati, D. Efficient isolation and enrichment of mesenchymal stem cells from bone marrow. Cytotherapy 2012, 14, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Zannetti, A.; Iommelli, F.; Fonti, R.; Papaccioli, A.; Sommella, J.; Lettieri, A.; Pirozzi, G.; Bianco, R.; Tortora, G.; Salvatore, M.; et al. Gefitinib induction of in vivo detectable signals by Bcl-2/Bcl-xL modulation of inositol trisphosphate receptor type 3. Clin. Cancer Res. 2008, 15, 5209–5219. [Google Scholar] [CrossRef] [PubMed]
- Geback, T.; Schulz, M.M.; Koumoutsakos, P.; Detmar, M. TScratch: A novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques 2009, 46, 265–274. [Google Scholar] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelagalli, A.; Nardelli, A.; Fontanella, R.; Zannetti, A. Inhibition of AQP1 Hampers Osteosarcoma and Hepatocellular Carcinoma Progression Mediated by Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2016, 17, 1102. https://doi.org/10.3390/ijms17071102
Pelagalli A, Nardelli A, Fontanella R, Zannetti A. Inhibition of AQP1 Hampers Osteosarcoma and Hepatocellular Carcinoma Progression Mediated by Bone Marrow-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2016; 17(7):1102. https://doi.org/10.3390/ijms17071102
Chicago/Turabian StylePelagalli, Alessandra, Anna Nardelli, Raffaela Fontanella, and Antonella Zannetti. 2016. "Inhibition of AQP1 Hampers Osteosarcoma and Hepatocellular Carcinoma Progression Mediated by Bone Marrow-Derived Mesenchymal Stem Cells" International Journal of Molecular Sciences 17, no. 7: 1102. https://doi.org/10.3390/ijms17071102
APA StylePelagalli, A., Nardelli, A., Fontanella, R., & Zannetti, A. (2016). Inhibition of AQP1 Hampers Osteosarcoma and Hepatocellular Carcinoma Progression Mediated by Bone Marrow-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences, 17(7), 1102. https://doi.org/10.3390/ijms17071102