Edwardsiella tarda Outer Membrane Protein C: An Immunogenic Protein Induces Highly Protective Effects in Flounder (Paralichthys olivaceus) against Edwardsiellosis
Abstract
:1. Introduction
2. Results
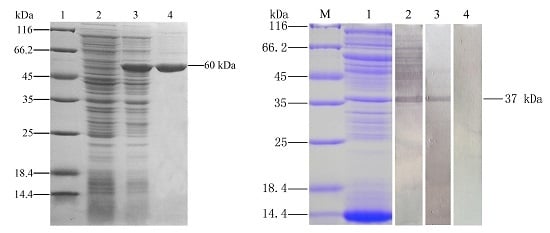
2.1. Expression and Purification of Recombinant Outer Membrane Protein C
2.2. Analysis of the Immunogenicity of OmpC
2.3. Flow Cytometric Immunofluorescence Analysis
2.4. Enzyme Linked Immunosorbent Analysis of Sera Antibodies against E. tarda
2.5. Immune Protective Effects of Recombinant Outer Membrane Protein C
2.6. Expression of Immune-Related Genes
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of Recombinant Outer Membrane Protein C
4.2. Preparation of Flounder Anti-rOmpC and Anti-E. tarda Antibodies
4.3. Analysis of the Immunogenicity of OmpC
4.4. Vaccination and Sampling
4.5. Flow Cytometric Immunofluorescence Analysis
4.6. Detection of the Serum Antibodies against E. tarda by ELISA
4.7. Analysis of the Expression of Immune-Related Genes by qRT-PCR
4.8. Challenge
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, C.; Hu, Y.H.; Chi, H.; Sun, L. The major fimbrial subunit protein of Edwardsiella tarda: Vaccine potential, adjuvant effect, and involvement in host infection. Fish Shellfish Immun. 2013, 35, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.Y.; Siame, B.A.; Tenkink, B.J.; Noort, R.J.; Mok, Y.K. Edwardsiella tarda—Virulence mechanisms of an emerging gastroenteritis pathogen. Microbes Infect. 2013, 14, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Kamaishi, T.; Ooseko, N.; Kurohara, K.; Lida, T. Pathogenicity of motile and non-motile Edwardsiella tarda to some marine fish. Fish. Pathol. 2005, 40, 133–135. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, H.Z.; Wang, Q.Y.; Qu, J.B.; Liu, Q.; Xiao, J.F.; Zhang, Y.X. A live attenuated combination vaccine evokes effective immune-mediated protection against Edwardsiella tarda and Vibrio anguillarum. Vaccine 2014, 32, 5937–5944. [Google Scholar] [CrossRef] [PubMed]
- Toranzo, A.E.; Magariños, B.; Romalde, J.L. A review of the main bacterial fish diseases in mariculture systems. Aquaculture 2005, 246, 37–61. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, X.H. Edwardsiella tarda: An intriguing problem in aquaculture. Aquaculture 2014, 431, 129–135. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, W.W.; Hou, J.H.; Sun, L. Immunoprotective analysis of VhhP2, a Vibrio harveyi vaccine candidate. Vaccine 2009, 27, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Arockiasamy, A.; Murthy, G.S.; Rukmini, M.R.; Sundara Baalaji, N.; Katpally, U.C.; Krishnaswamy, S. Conformational epitope mapping of OmpC, a major cell surface antigen from Salmonella typhi. J. Struct. Biol. 2004, 148, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.D.; Krishnaswamy, S. Overexpression, refolding, and purification of the major immunodominant outer membrane porin OmpC from Salmonella typhi: Characterization of refolded OmpC. Protein Express. Purif. 2005, 40, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Puente, J.L.; Alvarez-Scherer, V.; Gosset, G.; Calva, E. Comparative analysis of the Salmonella typhi and Escherichia coli OmpC genes. Gene 1989, 83, 197–206. [Google Scholar] [CrossRef]
- Puente, J.L.; Juárez, D.; Bobadilla, M.; Arias, C.F.; Calva, E. The Salmonella OmpC gene: Structure and use as a carrier for heterologous sequences. Gene 1995, 156, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Guan, Q.F.; Wang, X.M.; Teng, D.; Mao, R.Y.; Yao, J.H. Paving the way to construct a new vaccine against Escherichia coli from its recombinant outer membrane protein C via a murine model. Process. Biochem. 2015, 50, 1194–1201. [Google Scholar] [CrossRef]
- Agarwal, R.K.; Porteen, K.; Dubal, Z.B.; Asha, K.; Shweta, S.; Ripan, B. Evaluation of recombinant outer membrane protein based vaccine against Salmonella Typhimurium in birds. Biologicals 2013, 41, 162–168. [Google Scholar]
- Lee, H.; Kim, S.; Kim, K.; Mo, I.; Woo, Y.; Kwon, Y. Immunogenicity of outer membrane protein extracted from Salmonella gallinarum in chickens. Natl. Vet. Res. Inst. 1997, 37, 555–568. [Google Scholar]
- Jarząb, A.; Witkowska, D.; Ziomek, E.; Dabrowska, A.; Szewczuk, Z.; Gamain, A. Shigella flexneri 3a outer membrane protein C epitope is recognized by human umbilical cord sera and associated with protective activity. PLoS ONE 2013, 8, e70539. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Sahoo, P.K.; Dixit, A. Characterization of immune response elicited by the recombinant outer membrane protein OmpF of Aeromonas hydrophila, a potential vaccine candidate in murine model. Mol. Biol. Rep. 2014, 41, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.; Abbott, S. Infectious associated with the genus Edwardsiella: The role of Edwardsiella tarda in human disease. Clin. Infect. Dis. 1993, 17, 742–748. [Google Scholar] [PubMed]
- Park, S.B.; Aoki, T.; Jung, T.S. Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Vet. Res. 2012, 43, 67. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.J.; Zhan, W.B.; Ding, B.J.; Sheng, X.Z. Molecular cloning and expression analysis of polymeric immunoglobulin receptor in flounder (Paralichthys olivaceus). Fish Shellfish Immun. 2013, 35, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Oshima, S.; Kurohara, K.; Ohnishi, K.; Kawai, K. Vaccine efficacy of recombinant GAPDH of Edwardsiella tarda against edwardsiellosis. Microbiol. Immunol. 2005, 49, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Liu, Y.; Ohnishi, K.H.; Oshima, S.I. A conserved 37 kDa outer membrane protein of Edwardsiella tarda is an effective vaccine candidate. Vaccine 2004, 22, 3411–3418. [Google Scholar] [CrossRef] [PubMed]
- Pongprayoon, P. How do the protonation states of E296 and D312 in OmpF and D299 and D315 in homologous OmpC affect protein structure and dynamics? Simulation studies. Comput. Biol. Chem. 2014, 53, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hajjar, E.; Ruggerone, P.; Ceccarelli, M. Structural and dynamical properties of the porins OmpF and OmpC: Insights from molecular simulations. J. Phys. 2010, 22, 454125. [Google Scholar] [CrossRef] [PubMed]
- Biró, I.; Pezeshki, S.; Weingart, H.; Winterhalter, M.; Kleinekathöfer, U. Comparing the temperature-dependent conductance of the two structurally similar E. coli porins OmpC and OmpF. Biophys. J. 2010, 98, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Maiti, B.; Shetty, M.; Shekar, M.; Karunasagar, I.; Karunasagar, I. Recombinant outer membrane protein A (OmpA) of Edwardsiella tarda, a potential vaccine candidate for fish, common carp. Microbiol. Res. 2011, 167, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jeannin, P.; Magistrelli, G.; Goetsch, L.; Haeuw, J.F.; Thieblemont, N.; Bonnefoy, J.Y. Outer membrane protein A (OmpA): A new pathogen-associated molecular pattern that interacts with antigen presenting cells—Impact on vaccine strategies. Vaccine 2002, 20, 23–27. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Sun, L. Identification of an Edwardsiella tarda surface antigen and analysis of its immunoprotective potential as a purified recombinant subunit vaccine and a surface-anchored subunit vaccine expressed by a fish commensal strain. Vaccine 2010, 28, 6603–6608. [Google Scholar] [CrossRef] [PubMed]
- Croisetiere, S.; Tarte, P.D.; Bernatchez, L.; Belhumeur, P. Identification of MHC class IIβ resistance/susceptibility alleles to Aeromonas salmonicida in brook charr (Salvelinus fontinalis). Mol. Immunol. 2008, 45, 3107–3116. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, S.; Zhang, Y. MHC class IIα gene polymorphism and its association with resistance/susceptibility to Vibrio anguillarum in Japanese flounder (Paralichthys olivaceus). Dev. Comp. Immunol. 2010, 34, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Srisapoome, P.; Ohira, T.; Hirono, I.; AOKI, T. Cloning, characterization and expression of cDNA containing major histocompatibility complex class I, IIα and IIβ genes of Japanese flounder Paralichthys olivaceus. Fish. Sci. 2004, 70, 264–276. [Google Scholar] [CrossRef]
- Kjøglum, S.; Larsen, S.; Bakke, H.G.; Grimholt, U. How specific MHC class I and class II combinations affect disease resistance against infectious salmon anaemia in Atlantic salmon (Salmo salar). Fish Shellfish Immun. 2006, 21, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Pressley, M.E.; Phelan, P.E.; Witten, P.E.; Mellon, M.T.; Kim, C.H. Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Dev. Comp. Immunol. 2005, 29, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Taechavasonyoo, A.; Hirono, I.; Kondo, H. The immune-adjuvant effect of Japanese flounder Paralichthys olivaceus IL-1β. Dev. Comp. Immunol. 2013, 41, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Liu, Q.; Ni, C.S.; Li, S.; Wu, H.Z.; Wang, Q.Y.; Xiao, J.F.; Zhang, Y.X. Gene expression profiling in live attenuated Edwardsiella. tarda vaccine immunized and challenged zebrafish: Insights into the basic mechanisms of protection seen in immunized fish. Dev. Comp. Immunol. 2013, 40, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Aida, Y.; Pabst, M.J. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 1990, 132, 191–195. [Google Scholar] [CrossRef]
- Tang, X.Q.; Zhan, W.B.; Sheng, X.Z.; Chi, H. Immune response of Japanese flounder Paralichthys olivaceus to outer membrane protein of Edwardsiella tarda. Fish Shellfish Immun. 2010, 28, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Huntley, J.F.; Robertson, G.T.; Norgard, M.V. Method for the isolation of Francisella tularensis outer membranes. J. Vis. Exp. 2010, 40, e2044. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhan, W.B.; Xing, J.; Sheng, X.Z. Production, characterization and applicability of monoclonal antibodies to immunoglobulin of Japanese flounder (Paralichthys olivaceus). Fish Shellfish Immun. 2007, 23, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Amend, D.F. Potency testing of fish vaccines. International symposium on fish biologics: Serodiagnostics and vaccines. Dev. Biol. Stand. 1981, 49, 447–454. [Google Scholar]








| Number | Protein Name | Accession No. | Theoretical pI | Theoretical MW (Da) | Mascot Score /No. of Match Peptides | Protein Coverage (%) |
|---|---|---|---|---|---|---|
| 1 | Outer membrane protein A | gi|269138621 | 8.28 | 38057 | 350/4 | 19 |
| 2 | Outer membrane protein C | gi|387867288 | 5.26 | 40872 | 265/2 | 11 |
| 3 | Outer membrane porin F protein | gi|269138593 | 5.03 | 40058 | 429/3 | 19 |
| 4 | Glyceraldehyde-3-phosphate dehydrogenase | gi|550649563 | 6.60 | 35567 | 196/1 | 9 |
| Primer No. | Primer Name | Primer Sequence | Source |
|---|---|---|---|
| 1 | OmpC-F | 5′-CGAGCTC ATGATGAATAAAATCCGCTC-3’ | ETAE3470 |
| 2 | OmpC-R | 5’-CCGCTCGAGTTAGAACTTATAGTTCAGCATGG-3’ | |
| 3 | 18sRNA-F | 5’-GGTCTGTGATGCCCTTAGATGTC-3’ | EF126037 |
| 4 | 18sRNA-R | 5’-AGTGGGGTTCAGCGGGTTAC-3’ | |
| 5 | TLR2-F | 5’-CTGCGGTGTAGCGTTAGTGG-3’ | AB109393 |
| 6 | TLR2-R | 5’-CGAAGGCATCATAGGAAAGC-3’ | |
| 7 | TLR5M-F | 5’-TCCAGCATCATTACCAA-3’ | AB562152 |
| 8 | TLR5M-R | 5’-TCATACCCAAGTTAGCG-3’ | |
| 9 | IL-1β-F | 5’-CTGTCGTTCTGGGCATCAAA-3’ | AB720983 |
| 10 | IL-1β-R | 5’-AACAGAAATCGCACCATCTCACT-3’ | |
| 11 | TNFα-F | 5’-GTCCTGGCGTTTTCTTGGTA-3’ | AB040448 |
| 12 | TNFα-R | 5’-CTTGGCTCTGCTGCTGATTT-3’ | |
| 13 | IFN-γ-F | 5’-TGTCAGGTCAGAGGATCACACAT-3’ | AB435093 |
| 14 | IFN-γ-R | 5’-GCAGGAGGTTCTGGATGGTTT-3’ | |
| 15 | IL-6-F | 5’-CTCCGCAATGGGAAGGTG-3’ | DQ267937 |
| 16 | IL-6-R | 5’-GATGGATGGGTGGAATAA-3’ | |
| 17 | MHCΙα-F | 5’-AGACCACAGGCTGTTATCACCA-3’ | AB126921 |
| 18 | MHCΙα-R | 5’-TCTTCCCATGCTCCACGAA-3’ | |
| 19 | MHCΙΙα-F | 5’-ACAGGGACGGAACTTATCAACG-3’ | AY997530 |
| 20 | MHCΙΙα-R | 5’-TCATCGGACTGGAGGGAGG-3’ | |
| 21 | CD4-1-F | 5’-CCAGTGGTCCCCACCTAAAA-3’ | AB643634 |
| 22 | CD4-1-R | 5’-CACTTCTGGGACGGTGAGATG-3’ | |
| 23 | CD8α-F | 5’-CCTCTCCCCATACATTGATTCC-3’ | AB082957 |
| 24 | CD8α-R | 5’-CCGAGCTTTGCTGAAGGACTT-3’ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. Edwardsiella tarda Outer Membrane Protein C: An Immunogenic Protein Induces Highly Protective Effects in Flounder (Paralichthys olivaceus) against Edwardsiellosis. Int. J. Mol. Sci. 2016, 17, 1117. https://doi.org/10.3390/ijms17071117
Liu F, Tang X, Sheng X, Xing J, Zhan W. Edwardsiella tarda Outer Membrane Protein C: An Immunogenic Protein Induces Highly Protective Effects in Flounder (Paralichthys olivaceus) against Edwardsiellosis. International Journal of Molecular Sciences. 2016; 17(7):1117. https://doi.org/10.3390/ijms17071117
Chicago/Turabian StyleLiu, Fuguo, Xiaoqian Tang, Xiuzhen Sheng, Jing Xing, and Wenbin Zhan. 2016. "Edwardsiella tarda Outer Membrane Protein C: An Immunogenic Protein Induces Highly Protective Effects in Flounder (Paralichthys olivaceus) against Edwardsiellosis" International Journal of Molecular Sciences 17, no. 7: 1117. https://doi.org/10.3390/ijms17071117
APA StyleLiu, F., Tang, X., Sheng, X., Xing, J., & Zhan, W. (2016). Edwardsiella tarda Outer Membrane Protein C: An Immunogenic Protein Induces Highly Protective Effects in Flounder (Paralichthys olivaceus) against Edwardsiellosis. International Journal of Molecular Sciences, 17(7), 1117. https://doi.org/10.3390/ijms17071117