Genetic Mapping of a Major Resistance Gene to Pea Aphid (Acyrthosipon pisum) in the Model Legume Medicago truncatula
Abstract
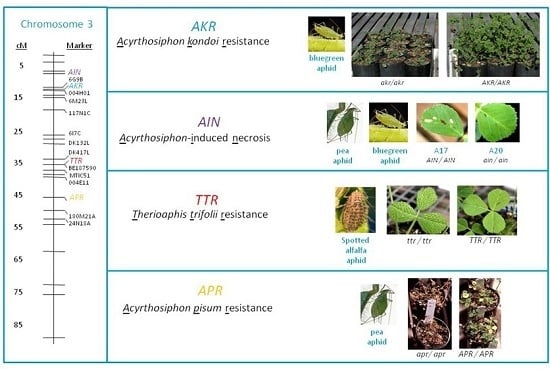
:1. Introduction
2. Results
2.1. Resistance to Pea Aphid in the Cultivar Jester Is Controlled by a Single Dominant Gene
2.2. Screening of M. truncatula Accessions for Additional Sources of PA Resistance
2.3. Resistance in M. truncatula Accessions SA10733 and SA10481 Is Controlled by Single Dominant Gene
3. Discussion
4. Materials and Methods
4.1. Plants and Aphids
4.2. Plant Damage and PA Performance Tests
4.3. Aphid Performance on Caged Leaves
4.4. Genetic Mapping of PA Resistance in the Various Mapping Populations
4.5. Allelism Tests
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ng, J.C.K.; Perry, K.L. Transmission of plant viruses by aphid vectors. Mol. Plant Pathol. 2004, 5, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Walling, L.L. Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol. 2008, 146, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Will, T.; Furch, A.C.; Zimmermann, M.R. How phloem-feeding insects face the challenge of phloem-located defenses. Front. Plant Sci. 2013, 4, 336. [Google Scholar] [CrossRef] [PubMed]
- Kaloshian, I. Gene-for-gene disease resistance: Bridging insect pest and pathogen defense. J. Chem. Ecol. 2004, 30, 2419–2438. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, L.G.; Zulak, K.; Gao, L.-L.; Anderson, J.P.; Singh, K.B. Plant-aphid interactions with a focus on legumes. Funct. Plant Biol. 2013, 40, 1271–1284. [Google Scholar] [CrossRef]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Eitas, T.K.; Dangl, J.L. NB–LRR proteins: Pairs, pieces, perception, partners and pathways. Curr. Opin. Plant Biol. 2010, 13, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; Walling, L.L.; Paine, T.D. Behavior and biology of the tomato psyllid, Bactericerca cockerelli, in response to the Mi-1.2 gene. Entomol. Exp. Appl. 2006, 121, 67–72. [Google Scholar] [CrossRef]
- Nombela, G.; Williamson, V.M.; Muñiz, M. The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol. Plant Microbe Interact. 2003, 16, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Goggin, F.; Milligan, S.B.; Kaloshian, I.; Ullman, D.E.; Williamson, V.M. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc. Natl. Acad. Sci. USA 1998, 95, 9750–9754. [Google Scholar] [CrossRef] [PubMed]
- Dogimont, C.; Chovelon, V.; Pauquet, J.; Boualem, A.; Bendahmane, A. The Vat locus encodes for a CC–NBS–LRR protein that confers resistance to Aphis gossypii infestation and A. gossypii-mediated virus resistance. Plant J. 2014, 80, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, T.; Piskurewicz, U.; Tomczak, A.; Ochoa, O.; Michelmore, R.W. Silencing of the major family of NBS–LRR encoding genes in lettuce results in the loss of multiple resistance specificities. Plant J. 2007, 51, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Klingler, J.; Creasy, R.; Gao, L.; Nair, R.M.; Calix, A.S.; Spafford Jacob, H.; Edwards, O.R.; Singh, K.B. Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS–LRR resistance gene analogs. Plant Physiol. 2005, 137, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Klingler, J.P.; Edwards, O.R.; Singh, K.B. Independent action and contrasting phenotypes of resistance genes against spotted alfalfa aphid and bluegreen aphid in Medicago truncatula. New Phytol. 2007, 173, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Klingler, J.P.; Nair, R.M.; Edwards, O.R.; Singh, K.B. A single gene, AIN, in Medicago truncatula mediates a hypersensitive response to both bluegreen aphid and pea aphid, but confers resistance only to bluegreen aphid. J. Exp. Bot. 2009, 60, 4115–4127. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Hodge, S.; Ismail, N.; Mansfield, J.W.; Feys, B.J.; Prosperi, J.M.; Huguet, T.; Ben, C.; Gentzbittel, L.; Powell, G. The RAP1 gene confers effective, race-specific resistance to the pea aphid in Medicago truncatula independent of the hypersensitive reaction. Mol. Plant Microbe Interact. 2009, 22, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Edwards, O.R.; Singh, K.B. Resistance to insect pests, what do legumes have to offer? Euphytica 2006, 147, 273–285. [Google Scholar] [CrossRef]
- Guo, S.; Kamphuis, L.G.; Gao, L.-L.; Edwards, O.R.; Singh, K.B. Two independent resistance genes in the Medicago truncatula cultivar Jester confer resistance to two different aphid species of the genus Acyrthosiphon. Plant Signal. Behav. 2009, 4, 1–4. [Google Scholar] [CrossRef]
- Guo, S.; Kamphuis, L.G.; Gao, L.-L.; Klingler, J.P.; Lichtenzveig, J.; Edwards, O.R.; Singh, K.B. Identification of distinct quantitative trait loci associated with defense against the closely related aphids Acyrthosiphon pisum and A. kondoi in Medicago truncatula. J. Exp. Bot. 2012, 63, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, L.G.; Gao, L.-L.; Singh, K.B. Identification and characterization of resistance to cowpea aphid (Aphis craccivora Koch) in Medicago truncatula. BMC Plant Biol. 2012, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, L.G.; Lichtenzveig, J.; Peng, K.; Guo, S.; Klingler, J.P.; Siddique, K.; Gao, L.L.; Singh, K.B. Physiology and genetics of resistance to spotted alfalfa aphid (Therioaphis trifolii) in Medicago truncatula. J. Exp. Bot. 2013, 64, 5157–5172. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.R. Jester. Plant Var. J. 2000, 13, 40. [Google Scholar]
- Gao, L.L.; Klingler, J.P.; Anderson, J.P.; Edwards, O.R.; Singh, K.B. Characterization of pea aphid resistance in Medicago truncatula. Plant Physiol. 2008, 146, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- IAGC. Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010, 8, e1000313. [Google Scholar] [CrossRef] [Green Version]
- Legeai, F.; Shigenobu, S.; Gauthier, J.P.; Colbourne, J.; Rispe, C.; Collin, O.; Richards, S.; Wilson, A.C.; Murphy, T.; Tagu, D. AphidBase: A centralized bioinformatic resource for annotation of the pea aphid genome. Insect Mol. Biol. 2010, 2, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Kanvil, S.; Powell, G.; Turnbull, C. Pea aphid biotype performance on diverse Medicago host genotypes indicates highly specific virulence and resistance functions. Bull. Entomol. Res. 2014, 104, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Kim, D.; Uhm, T.; Limpens, E.; Lim, H.; Mun, J.H.; Kalo, P.; Penmetsa, R.V.; Seres, A.; Kulikova, O.; et al. A sequence-based genetic map of Medicago truncatula and comparison of marker colinearity with M. sativa. Genetics 2004, 166, 1463–1502. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.V.; Vaz Patto, M.C.; Huguet, T.; Cubero, J.I.; Moreno, M.T.; Torres, A.M. Cross-species amplification of Medicago truncatula microsatellites across three major pulse crops. Theor. Appl. Genet. 2005, 110, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.H.; Kim, D.J.; Choi, H.K.; Gish, J.; Debellé, F.; Mudge, J.; Denny, R.; Endré, S.; Saurat, O.; Dudez, A.M.; et al. Distribution of microsatellites in the genome of Medicago truncatula: A resource of genetic markers that integrate genetic and physical maps. Genetics 2006, 172, 2541–2555. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, S.R.; D’Souza, N.K.; Kamphuis, L.G.; Burgess, T.I.; Nair, R.M.; Oliver, R.P. SSR analysis of the Medicago truncatula SARDI core collection reveals substantial diversity and unusual genotype dispersal throughout the Mediterranean basin. Theor. Appl. Genet. 2006, 112, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.L.; Horbury, R.; Nair, R.M.; Edwards, O.R.; Singh, K.B. Characterization of resistance to multiple aphid species (Hemiptera: Aphididae) in Medicago truncatula. Bull. Entomol. Res. 2007, 97, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Sakiroglu, M.; Krom, N.; Stanton-Geddes, J.; Wang, M.; Lee, Y.C.; Young, N.D.; Udvardi, M. Genome-wide association of drought-related and biomass traits with HapMap SNPs in Medicago truncatula. Plant Cell Environ. 2015, 38, 1997–2011. [Google Scholar] [CrossRef] [PubMed]
- Kanvil, S.; Collins, C.M.; Powell, G.; Turnbull, C.G.N. Cryptic virulence and avirulence alleles revealed by controlled sexual recombination in pea aphids. Genetics 2015, 199, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Michelmore, R.W.; Meyers, B.C. Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res. 1998, 8, 1113–1130. [Google Scholar] [PubMed]
- Tang, H.; Krishnakumar, V.; Bidwell, S.; Rosen, B.; Chan, A.; Zhou, S.; Gentzbittel, L.; Childs, K.L.; Yandell, M.; Gundlach, H.; et al. An improved genome release (version Mt4.0) for the model legume Medicago truncatula. BMC Genom. 2014, 15, 312. [Google Scholar] [CrossRef] [PubMed]
- Young, N.D.; Debellé, F.; Oldroyd, G.E.; Geurts, R.; Cannon, S.B.; Udvardi, M.K.; Benedito, V.A.; Mayer, K.F.; Gouzy, J.; Schoof, H.; et al. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 2011, 480, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Gerardo, N.M.; Altincicek, B.; Anselme, C.; Atamian, H.; Barribeau, S.M.; de Vos, M.; Duncan, E.I.; Evans, J.D.; Gabaldon, T.; Ghanim, M.; et al. Immunity and other defenses in pea aphids, acyrthosiphon pisum. Genome Biol. 2010, 11, R21. [Google Scholar] [CrossRef] [PubMed]
- Mutti, N.S.; Louis, J.; Pappan, L.K.; Pappan, K.; Begum, K.; Chen, M.-S.; Park, Y.; Dittmer, N.; Marshall, J.; Reese, J.C.; et al. A protein from the salivary glands of the pea aphid, acyrthosiphon pisum, is essential in feeding on a host plant. Proc. Natl. Acad. Sci. USA 2008, 105, 9965–9969. [Google Scholar] [CrossRef] [PubMed]
- Mutti, N.S.; Park, Y.; Reese, J.C.; Reeck, G.R. RNAi knockdown of a salivary transcript leading to lethality in the pea aphid Acyrthosiphon pisum. J. Insect Sci. 2006, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Thoquet, P.; Ghérardi, M.; Journet, E.; Kereszt, A.; Ané, J.; Prosperi, J.M.; Huguet, T. The molecular genetic linkage map of the model legume Medicago truncatula: An essential tool for comparative legume genomics and the isolation of agronomically important genes. BMC Plant Biol. 2002, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamphuis, L.G.; Lichtenzveig, J.; Oliver, R.P.; Ellwood, S.R. Two alternative recessive quantitative trait loci influence resistance to spring black stem and leaf spot in Medicago truncatula. BMC Plant Biol. 2008, 8, 1. [Google Scholar] [CrossRef] [PubMed]



| Accession | Aphid Score 9 dpi (SE) | Plant Score 15 dpi (SE) | Plant Score 21 dpi (SE) | Plant Survivorship 28 dpi | Comment |
|---|---|---|---|---|---|
| SA11753 | 2.5(0.3) | 1.6(0.7) | 3.0(0.0) | 3/3 | Resistant |
| SA28645 | 2.5(0.9) | 2.2(0.3) | 3.0(0.1) | 3/3 | Resistant |
| SA3047 | 1.8(0.6) | 2.5(0.3) | 3.1(0.1) | 3/3 | Resistant |
| SA10481 | 2.8(0.4) | 2.3(0.3) | 3.3(0.2) | 3/3 | Resistant |
| SA1516 | 1.7(0.2) | 2.5(0.3) | 3.6(0.5) | 3/3 | Resistant |
| SA27192 | 1.7(0.2) | 1.3(0.1) | 3.6(0.6) | 3/3 | Resistant |
| SA27063 | 2.3(0.3) | 3.5(0.3) | 3.6(0.4) | 2/3 | Resistant |
| Caliph | 1.8(0.3) | 2.4(0.3) | 3.8(0.4) | 3/3 | Resistant (control) |
| Jester | 1.5(0.3) | 2.7(0.2) | 3.9(0.2) | 3/3 | Resistant (control) |
| SA25654 | 2.3(0.3) | 2.0(0.6) | 3.3(0.3) | 0/3 | Moderately susceptible |
| SA18395 | 1.2(0.2) | 2.1(0.6) | 3.5(0.0) | 0/3 | Moderately susceptible |
| SA8604 | 2.3(0.2) | 2.0(0.6) | 4.0(0.4) | 0/3 | Moderately susceptible |
| SA9062 | 2.3(0.3) | 2.3(0.3) | 4.0(0.4) | 0/3 | Moderately susceptible |
| SA30199 | 2.2(0.4) | 2.3(0.2) | 4.1(0.2) | 0/3 | Moderately susceptible |
| SA3569 | 2.2(0.4) | 2.2(0.3) | 4.3(0.3) | 0/3 | Moderately susceptible |
| SA10419 | 2.7(0.7) | 4.3(0.6) | 4.4(0.6) | 0/3 | Moderately susceptible |
| DZA315 | 2.8(0.6) | 4.0(0.1) | 4.6(0.1) | 0/3 | Moderately susceptible |
| SA17590 | 2.8(0.7) | 3.0(0.6) | 4.6(0.2) | 0/3 | Moderately susceptible |
| A17 | 2.7(0.2) | 3.0(0.5) | 4.7(0.1) | 0/3 | Moderately susceptible (control) |
| SA3919 | 1.7(0.4) | 2.3(0.6) | 4.7(0.2) | 0/3 | Susceptible |
| SA24968 | 2.2(0.4) | 3.3(0.7) | 4.8(0.1) | 0/3 | Susceptible |
| SA3054 | 2.8(0.4) | 2.9(0.9) | 4.8(0.1) | 0/3 | Susceptible |
| SA8618 | 3.2(0.2) | 3.3(0.3) | 4.8(0.3) | 0/3 | Susceptible |
| SA11734 | 2.5(0.3) | 4.1(0.5) | 4.9(0.1) | 0/3 | Susceptible |
| SA9357 | 3.8(0.2) | 4.3(0.2) | 4.9(0.1) | 0/3 | Susceptible |
| SA22323 | 3.3(0.3) | 4.3(0.3) | 5.0(0.0) | 0/3 | Susceptible |
| SA7749 | 3.2(0.3) | 4.6(0.1) | 5.0(0.0) | 0/3 | Susceptible |
| SA9710 | 2.5(0.3) | 4.5(0.3) | 5.0(0.0) | 0/3 | Susceptible |
| SA9712 | 2.7(0.2) | 4.4(0.2) | 5.0(0.0) | 0/3 | Susceptible |
| Cyprus | 3.0(0.6) | 4.5(0.3) | 5.0(0.0) | 0/3 | Susceptible (control) |
| Borung | 3.3(0.2) | 4.9(0.1) | 5.0(0.0) | 0/3 | Susceptible (control) |
| A20 | 3.7(0.2) | 4.8(0.1) | 5.0(0.0) | 0/3 | Susceptible (control) |
| SA1499 | 3.3(0.2) | 4.4(0.2) | 5.0(0.0) | 0/3 | Susceptible |
| DZA045 | 3.0(0.5) | 4.8(0.1) | 5.0(0.0) | 0/3 | Susceptible |
| SA1489 | 3.7(0.2) | 4.9(0.1) | 5.0(0.0) | 0/3 | Susceptible |
| Population | Resistant: Susceptible | |||
|---|---|---|---|---|
| Observed | Expected | χ2 | p | |
| SA10733 × A20 | 200:64 | 198:66 | 0.081 | 0.776 |
| SA10481 × A20 | 264:91 | 266:89 | 0.076 | 0.783 |
| Population | Resistant: Susceptible | |||
|---|---|---|---|---|
| Observed | Expected | χ2 | p | |
| SA10733 × SA1516 | 144:0 | 135:9 | 9.6 | 0.0019 |
| SA1516 × SA10481 | 100:0 | 93.75:6.25 | 6.667 | 0.010 |
| SA10481 × SA10733 | 250:0 | 234.4:15.6 | 16.667 | 0.00005 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamphuis, L.G.; Guo, S.-M.; Gao, L.-L.; Singh, K.B. Genetic Mapping of a Major Resistance Gene to Pea Aphid (Acyrthosipon pisum) in the Model Legume Medicago truncatula. Int. J. Mol. Sci. 2016, 17, 1224. https://doi.org/10.3390/ijms17081224
Kamphuis LG, Guo S-M, Gao L-L, Singh KB. Genetic Mapping of a Major Resistance Gene to Pea Aphid (Acyrthosipon pisum) in the Model Legume Medicago truncatula. International Journal of Molecular Sciences. 2016; 17(8):1224. https://doi.org/10.3390/ijms17081224
Chicago/Turabian StyleKamphuis, Lars G., Su-Min Guo, Ling-Ling Gao, and Karam B. Singh. 2016. "Genetic Mapping of a Major Resistance Gene to Pea Aphid (Acyrthosipon pisum) in the Model Legume Medicago truncatula" International Journal of Molecular Sciences 17, no. 8: 1224. https://doi.org/10.3390/ijms17081224
APA StyleKamphuis, L. G., Guo, S. -M., Gao, L. -L., & Singh, K. B. (2016). Genetic Mapping of a Major Resistance Gene to Pea Aphid (Acyrthosipon pisum) in the Model Legume Medicago truncatula. International Journal of Molecular Sciences, 17(8), 1224. https://doi.org/10.3390/ijms17081224