Association between IRS1 Gene Polymorphism and Autism Spectrum Disorder: A Pilot Case-Control Study in Korean Males
Abstract
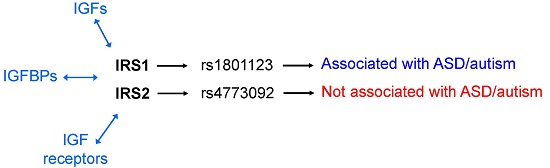
:1. Introduction
2. Results
3. Discussion
4. Experimental Section
4.1. Subjects
4.2. Single-Nucleotide Polymorphism (SNP) Selection and Genotyping
4.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tanguay, P.E. Pervasive developmental disorders: A 10-year review. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 1079–1095. [Google Scholar]
- Nicholas, J.S.; Charles, J.M.; Carpenter, L.A.; King, L.B.; Jenner, W.; Spratt, E.G. Prevalence and characteristics of children with autism-spectrum disorders. Ann. Epidemiol. 2008, 18, 130–136. [Google Scholar]
- Burmeister, M.; McInnis, M.G.; Zollner, S. Psychiatric genetics: Progress amid controversy. Nat. Rev. Genet. 2008, 9, 527–540. [Google Scholar]
- Nowakowska, B.A.; de Leeuw, N.; Ruivenkamp, C.A.; Sikkema-Raddatz, B.; Crolla, J.A.; Thoelen, R.; Koopmans, M.; den Hollander, N.; van Haeringen, A.; van der Kevie-Kersemaekers, A.M.; et al. Parental insertional balanced translocations are an important cause of apparently de novo CNVs in patients with developmental anomalies. Eur. J. Hum. Genet. 2012, 20, 166–170. [Google Scholar]
- Carter, M.T.; Nikkel, S.M.; Fernandez, B.A.; Marshall, C.R.; Noor, A.; Lionel, A.C.; Prasad, A.; Pinto, D.; Joseph-George, A.M.; Noakes, C.; et al. Hemizygous deletions on chromosome 1p21.3 involving the DPYD gene in individuals with autism spectrum disorder. Clin. Genet. 2011, 80, 435–443. [Google Scholar]
- Anney, R.; Klei, L.; Pinto, D.; Regan, R.; Conroy, J.; Magalhaes, T.R.; Correia, C.; Abrahams, B.S.; Sykes, N.; Pagnamenta, A.T.; et al. A genome-wide scan for common alleles affecting risk for autism. Hum. Mol. Genet. 2010, 19, 4072–4082. [Google Scholar] [Green Version]
- Weiss, L.A.; Arking, D.E. Gene Discovery Project of Johns Hopkins and the Autism Consortium. A genome-wide linkage and association scan reveals novel loci for autism. Nature 2009, 461, 802–808. [Google Scholar] [PubMed]
- Wang, K.; Zhang, H.; Ma, D.; Bucan, M.; Glessner, J.T.; Abrahams, B.S.; Salyakina, D.; Imielinski, M.; Bradfield, J.P.; Sleiman, P.M.; et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 2009, 459, 528–533. [Google Scholar]
- Chen, D.Y.; Stern, S.A.; Garcia-Osta, A.; Saunier-Rebori, B.; Pollonini, G.; Bambah-Mukku, D.; Blitzer, R.D.; Alberini, C.M. A critical role for IGF-II in memory consolidation and enhancement. Nature 2011, 469, 491–497. [Google Scholar]
- O’Kusky, J.R.; Ye, P.; D’Ercole, A.J. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J. Neurosci. 2000, 20, 8435–8442. [Google Scholar]
- Schaevitz, L.R.; Moriuchi, J.M.; Nag, N.; Mellot, T.J.; Berger-Sweeney, J. Cognitive and social functions and growth factors in a mouse model of Rett syndrome. Physiol. Behav. 2010, 100, 255–263. [Google Scholar]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Nonneman, R.J.; Grossman, A.W.; Murphy, D.L.; D’Ercole, A.J.; Crawley, J.N.; Magnuson, T.R.; Lauder, J.M. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009, 8, 129–142. [Google Scholar]
- Mills, J.L.; Hediger, M.L.; Molloy, C.A.; Chrousos, G.P.; Manning-Courtney, P.; Yu, K.F.; Brasington, M.; England, L.J. Elevated levels of growth-related hormones in autism and autism spectrum disorder. Clin. Endocrinol. 2007, 67, 230–237. [Google Scholar]
- Courchesne, E.; Carper, R.; Akshoomoff, N. Evidence of brain overgrowth in the first year of life in autism. JAMA 2003, 290, 337–344. [Google Scholar]
- Courchesne, E.; Karns, C.M.; Davis, H.R.; Ziccardi, R.; Carper, R.A.; Tigue, Z.D.; Chisum, H.J.; Moses, P.; Pierce, K.; Lord, C.; et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology 2001, 57, 245–254. [Google Scholar]
- Popken, G.J.; Hodge, R.D.; Ye, P.; Zhang, J.; Ng, W.; O’Kusky, J.R.; D’Ercole, A.J. In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system. Eur. J. Neurosci. 2004, 19, 2056–2068. [Google Scholar]
- Riikonen, R. Neurotrophic factors in the pathogenesis of Rett syndrome. J. Child Neurol. 2003, 18, 693–697. [Google Scholar]
- Vanhala, R.; Turpeinen, U.; Riikonen, R. Low levels of insulin-like growth factor-I in cerebrospinal fluid in children with autism. Dev. Med. Child Neurol. 2001, 43, 614–616. [Google Scholar]
- Steinman, G.; Mankuta, D. Insulin-like growth factor and the etiology of autism. Med. Hypotheses 2013, 80, 475–480. [Google Scholar]
- Laban, C.; Bustin, S.A.; Jenkins, P.J. The GH-IGF-I axis and breast cancer. Trends Endocrinol. Metab. 2003, 14, 28–34. [Google Scholar]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar]
- White, M.F. Insulin signaling in health and disease. Science 2003, 302, 1710–1711. [Google Scholar]
- Burks, D.J.; White, M.F. IRS proteins and β-cell function. Diabetes 2001, 50, S140–S145. [Google Scholar]
- Withers, D.J.; Burks, D.J.; Towery, H.H.; Altamuro, S.L.; Flint, C.L.; White, M.F. IRS-2 coordinates IGF-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat. Genet. 1999, 23, 32–40. [Google Scholar]
- Werner, H.; Le Roith, D. The insulin-like growth factor-I receptor signaling pathways are important for tumorigenesis and inhibition of apoptosis. Crit. Rev. Oncog. 1997, 8, 71–92. [Google Scholar]
- Van Obberghen, E.; Baron, V.; Scimeca, J.C.; Kaliman, P. Insulin receptor: Receptor activation and signal transduction. Adv. Second Messenger Phosphoprot. Res. 1993, 28, 195–201. [Google Scholar]
- Feng, X.; Tucker, K.L.; Parnell, L.D.; Shen, J.; Lee, Y.C.; Ordovas, J.M.; Ling, W.H.; Lai, C.Q. Insulin receptor substrate 1 (IRS1) variants confer risk of diabetes in the Boston Puerto Rican Health Study. Asia Pac. J. Clin. Nutr. 2013, 22, 150–159. [Google Scholar] [PubMed]
- Lautier, C.; El Mkadem, S.A.; Renard, E.; Brun, J.F.; Gris, J.C.; Bringer, J.; Grigorescu, F. Complex haplotypes of IRS2 gene are associated with severe obesity and reveal heterogeneity in the effect of Gly1057Asp mutation. Hum. Genet. 2003, 113, 34–43. [Google Scholar]
- Winder, T.; Giamas, G.; Wilson, P.M.; Zhang, W.; Yang, D.; Bohanes, P.; Ning, Y.; Gerger, A.; Stebbing, J.; Lenz, H.J. Insulin-like growth factor receptor polymorphism defines clinical outcome in estrogen receptor-positive breast cancer patients treated with tamoxifen. Pharmacogen. J. 2014, 14, 28–34. [Google Scholar]
- Neuhausen, S.L.; Brummel, S.; Ding, Y.C.; Singer, C.F.; Pfeiler, G.; Lynch, H.T.; Nathanson, K.L.; Rebbeck, T.R.; Garber, J.E.; Couch, F.; et al. Genetic variation in insulin-like growth factor signaling genes and breast cancer risk among BRCA1 and BRCA2 carriers. Breast Cancer Res. 2009, 11, R76. [Google Scholar]
- Slattery, M.L.; Samowitz, W.; Curtin, K.; Ma, K.N.; Hoffman, M.; Caan, B.; Neuhausen, S. Associations among IRS1, IRS2, IGF1, and IGFBP3 genetic polymorphisms and colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1206–1214. [Google Scholar]
- Genetic Power Calculator. Available online: http://pngu.mgh.harvard.edu/~purcell/gpc/cc2.html (accessed on 28 July 2016).
- Rasmussen, S.K.; Urhammer, S.A.; Hansen, T.; Almind, K.; Moller, A.M.; Borch-Johnsen, K.; Pedersen, O. Variability of the insulin receptor substrate-1, hepatocyte nuclear factor-1α (HNF-1α), HNF-4α, and HNF-6 genes and size at birth in a population-based sample of young Danish subjects. J. Clin. Endocrinol. Metab. 2000, 85, 2951–2953. [Google Scholar]
- Almind, K.; Frederiksen, S.K.; Bernal, D.; Hansen, T.; Ambye, L.; Urhammer, S.; Ekstrom, C.T.; Berglund, L.; Reneland, R.; Lithell, H.; et al. Search for variants of the gene-promoter and the potential phosphotyrosine encoding sequence of the insulin receptor substrate-2 gene: Evaluation of their relation with alterations in insulin secretion and insulin sensitivity. Diabetologia 1999, 42, 1244–1249. [Google Scholar]
- Almind, K.; Bjorbaek, C.; Vestergaard, H.; Hansen, T.; Echwald, S.; Pedersen, O. Aminoacid polymorphisms of insulin receptor substrate-1 in non-insulin-dependent diabetes mellitus. Lancet 1993, 342, 828–832. [Google Scholar]
- Withers, D.J.; Gutierrez, J.S.; Towery, H.; Burks, D.J.; Ren, J.M.; Previs, S.; Zhang, Y.; Bernal, D.; Pons, S.; Shulman, G.I.; et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 1998, 391, 900–904. [Google Scholar]
- Tamemoto, H.; Kadowaki, T.; Tobe, K.; Yagi, T.; Sakura, H.; Hayakawa, T.; Terauchi, Y.; Ueki, K.; Kaburagi, Y.; Satoh, S.; et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 1994, 372, 182–186. [Google Scholar]
- Anlar, B.; Sullivan, K.A.; Feldman, E.L. Insulin-like growth factor-I and central nervous system development. Horm. Metab. Res. 1999, 31, 120–125. [Google Scholar]
- Onuma, T.A.; Ding, Y.; Abraham, E.; Zohar, Y.; Ando, H.; Duan, C. Regulation of temporal and spatial organization of newborn GnRH neurons by IGF signaling in zebrafish. J. Neurosci. 2011, 31, 11814–11824. [Google Scholar]
- Beck, K.D.; Powell-Braxton, L.; Widmer, H.R.; Valverde, J.; Hefti, F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 1995, 14, 717–730. [Google Scholar]
- Ding, Y.C.; McGuffog, L.; Healey, S.; Friedman, E.; Laitman, Y.; Paluch-Shimon, S.; Kaufman, B.; Swe, B.; Liljegren, A.; Lindblom, A.; et al. A nonsynonymous polymorphism in IRS1 modifies risk of developing breast and ovarian cancers in BRCA1 and ovarian cancer in BRCA2 mutation carriers. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1362–1370. [Google Scholar]
- Association Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Press: Washington, DC, USA, 1994. [Google Scholar]
- Schopler, E.; Reichier, R.J.; Renner, B.R. Childhood Autism Rating Scale; Western Psychological Services: Los Angeles, CA, USA, 1988. [Google Scholar]
- NCBI databases. Available online: http://www.ncbi.nlm.nih.gov/SNP (accessed on 28 July 2016).
- Collins, F.S.; Guyer, M.S.; Chakravarti, A. Variations on a theme: Cataloging human DNA sequence variations. Science 1997, 278, 1580–1581. [Google Scholar]
- Garg, K.; Green, P.; Nickerson, D.A. Identification of candidate coding region single nucleotide polymorphisms in 165 human genes using assembled expressed sequence tags. Genome Res. 1999, 9, 1087–1092. [Google Scholar]
- ICO. Available online: http://bioinfo.iconcologia.net/index.php (accessed on 28 July 2016).
| SNP | Model/Allele | Genotype | Control | ASD | OR (95% CI) | p |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| rs1801123 | Additive | AA | 73 (49.7) | 114 (63.3) | 1 | |
| Ala804Ala | AG | 63 (42.9) | 56 (31.1) | |||
| IRS1 | GG | 11 (7.5) | 10 (5.6) | 0.66 (0.46–0.95) | 0.022 | |
| Dominant | AA | 73 (49.7) | 114 (63.3) | 1 | ||
| AG/GG | 74 (50.3) | 66 (36.7) | 0.57 (0.37–0.89) | 0.013 | ||
| Recessive | AA/AG | 136 (92.5) | 170 (94.4) | 1 | ||
| GG | 11 (7.5) | 10 (5.6) | 0.73 (0.30–1.76) | 0.48 | ||
| Allele | A | 209 (71.1) | 284 (78.9) | 1 | ||
| G | 85 (28.9) | 76 (21.1) | 0.66 (0.46–0.94) | 0.022 | ||
| rs4773092 | Additive | AA | 41 (27.9) | 51 (28.3) | 1 | |
| Cys816Cys | AG | 76 (51.7) | 95 (52.8) | |||
| IRS2 | GG | 30 (20.4) | 34 (18.9) | 0.96 (0.70–1.32) | 0.8 | |
| Dominant | AA | 41 (27.9) | 51 (28.3) | 1 | ||
| AG/GG | 106 (72.1) | 129 (71.7) | 0.98 (0.60–1.59) | 0.93 | ||
| Recessive | AA/AG | 117 (79.6) | 146 (81.1) | 1 | ||
| GG | 30 (20.4) | 34 (18.9) | 0.91 (0.53–1.57) | 0.73 | ||
| Allele | A | 158 (53.7) | 197 (54.7) | 1 | ||
| G | 136 (46.3) | 163 (45.3) | 0.96 (0.70–1.31) | 0.8 |
| SNP | Model/allele | Genotype | Control | Autistic Disorder | OR (95% CI) | p |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| rs1801123 | Additive | AA | 73 (49.7) | 91 (66.4) | 1 | |
| Ala804Ala | AG | 63 (42.9) | 41 (29.9) | |||
| IRS1 | GG | 11 (7.5) | 5 (3.6) | 0.56 (0.37–0.83) | 0.0037 | |
| Dominant | AA | 73 (49.7) | 91 (66.4) | 1 | ||
| AG/GG | 74 (50.3) | 46 (33.6) | 0.50 (0.31–0.81) | 0.0041 | ||
| Recessive | AA/AG | 136 (92.5) | 132 (96.3) | 1 | ||
| GG | 11 (7.5) | 5 (3.6) | 0.47 (0.16–1.38) | 0.16 | ||
| Allele | A | 209 (71.1) | 223 (81.4) | 1 | ||
| G | 85 (28.9) | 51 (18.6) | 0.56 (0.38–0.84) | 0.004 | ||
| rs4773092 | Additive | AA | 41 (27.9) | 36 (26.3) | 1 | |
| Cys816Cys | AG | 76 (51.7) | 70 (51.1) | |||
| IRS2 | GG | 30 (20.4) | 31 (22.6) | 1.08 (0.77–1.51) | 0.64 | |
| Dominant | AA | 41 (27.9) | 36 (26.3) | 1 | 0.76 | |
| AG/GG | 106 (72.1) | 101 (73.7) | 1.09 (0.64–1.83) | |||
| Recessive | AA/AG | 117 (79.6) | 106 (77.4) | 1 | 0.65 | |
| GG | 30 (20.4) | 31 (22.6) | 1.14 (0.65–2.01) | |||
| Allele | A | 158 (53.7) | 142 (51.8) | 1 | ||
| G | 136 (46.3) | 132 (48.2) | 0.96 (0.70–1.31) | 0.8 |
| Characteristics | ASDs | Control |
|---|---|---|
| Total no. of subject | 180 | 147 |
| Age (mean ± SD, years) | 15.5 ± 4.8 | 39.9 ± 5.8 |
| CARS score | 38.6 ± 5.8 | |
| Autistic disorder (n = 137) | 41.1 ± 4.3 | |
| Asperger‘s disorder (n = 11) | 30.7 ± 0.4 | |
| PDD-NOS (n = 32) | 30.9 ± 1.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.J.; Kim, S.K.; Kang, W.S.; Park, J.K.; Kim, Y.J.; Nam, M.; Kim, J.W.; Chung, J.-H. Association between IRS1 Gene Polymorphism and Autism Spectrum Disorder: A Pilot Case-Control Study in Korean Males. Int. J. Mol. Sci. 2016, 17, 1227. https://doi.org/10.3390/ijms17081227
Park HJ, Kim SK, Kang WS, Park JK, Kim YJ, Nam M, Kim JW, Chung J-H. Association between IRS1 Gene Polymorphism and Autism Spectrum Disorder: A Pilot Case-Control Study in Korean Males. International Journal of Molecular Sciences. 2016; 17(8):1227. https://doi.org/10.3390/ijms17081227
Chicago/Turabian StylePark, Hae Jeong, Su Kang Kim, Won Sub Kang, Jin Kyung Park, Young Jong Kim, Min Nam, Jong Woo Kim, and Joo-Ho Chung. 2016. "Association between IRS1 Gene Polymorphism and Autism Spectrum Disorder: A Pilot Case-Control Study in Korean Males" International Journal of Molecular Sciences 17, no. 8: 1227. https://doi.org/10.3390/ijms17081227
APA StylePark, H. J., Kim, S. K., Kang, W. S., Park, J. K., Kim, Y. J., Nam, M., Kim, J. W., & Chung, J. -H. (2016). Association between IRS1 Gene Polymorphism and Autism Spectrum Disorder: A Pilot Case-Control Study in Korean Males. International Journal of Molecular Sciences, 17(8), 1227. https://doi.org/10.3390/ijms17081227