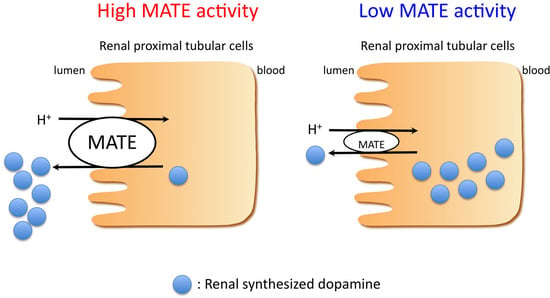
Urinary Dopamine as a Potential Index of the Transport Activity of Multidrug and Toxin Extrusion in the Kidney
Abstract
:1. Introduction
2. Results
2.1. Dopamine Transport Is Mediated by Multidrug and Toxin Extrusion (MATE)
2.2. Effects of Mate1 Knockout on Urinary Dopamine and Na+ Excretion in Mice
2.3. Effects of Imatinib on Urinary Dopamine and Na+ Excretion in Mice
2.4. Imatinib Inhibits MATE-Dependent Uptake of Dopamine
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Dopamine Uptake Experiments
4.3. Animals
4.4. Immunohistochemical Analysis
4.5. Determination of Fluid Content
4.6. Acute Saline Volume Expansion Experiments
4.7. Sample Preparation
4.8. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, C.J.; Lokhandwala, M.F. Role of endogenous dopamine in the natriuretic response to various degrees of iso-osmotic volume expansion in rats. Clin. Exp. Hypertens. A 1991, 13, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Yamaji, Y.; Kitajima, W.; Saruta, T. Effects of high salt intake on dopamine production in rat kidney. Am. J. Physiol. 1991, 260, E675–E679. [Google Scholar] [PubMed]
- Alexander, R.W.; Gill, J.R., Jr.; Yamabe, H.; Lovenberg, W.; Keiser, H.R. Effects of dietary sodium and of acute saline infusion on the interrelationship between dopamine excretion and adrenergic activity in man. J. Clin. Investig. 1974, 54, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Villar, V.A.; Armando, I.; Eisner, G.M.; Felder, R.A.; Jose, P.A. Dopamine, kidney, and hypertension: Studies in dopamine receptor knockout mice. Pediatr. Nephrol. 2008, 23, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Lokhandwala, M.F. An impairment of renal tubular DA-1 receptor function as the causative factor for diminished natriuresis to volume expansion in spontaneously hypertensive rats. Clin. Exp. Hypertens. A 1992, 14, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.G.; Oats, N.S.; Lee, M.R. Urinary dopamine in man and rat: Effects of inorganic salts on dopamine excretion. Clin. Sci. Mol. Med. 1978, 55, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Quinones, H.; Collazo, R.; Moe, O.W. The dopamine precursor l-dihydroxyphenylalanine is transported by the amino acid transporters rBAT and LAT2 in renal cortex. Am. J. Physiol. Ren. Physiol. 2004, 287, F74–F80. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.; Pinho, M.J.; Soares-da-Silva, P. Renal amino acid transport systems and essential hypertension. FASEB J. 2013, 27, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Yamaji, Y.; Kitajima, W.; Saruta, T. Aromatic l-amino acid decarboxylase activity along the rat nephron. Am. J. Physiol. 1990, 258, F28–F33. [Google Scholar] [PubMed]
- Jose, P.A.; Soares-da-Silva, P.; Eisner, G.M.; Felder, R.A. Dopamine and G protein-coupled receptor kinase 4 in the kidney: Role in blood pressure regulation. Biochim. Biophys. Acta 2010, 1802, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Terada, T.; Asaka, J.; Ueba, M.; Katsura, T.; Inui, K. Oppositely directed H+ gradient functions as a driving force of rat H+/organic cation antiporter MATE1. Am. J. Physiol. Ren. Physiol. 2007, 292, F593–F598. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Matsumoto, T.; Morimoto, R.; Arioka, S.; Omote, H.; Moriyama, Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc. Natl. Acad. Sci. USA 2005, 102, 17923–17928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, S.; Terada, T.; Yonezawa, A.; Tanihara, Y.; Kishimoto, K.; Katsura, T.; Ogawa, O.; Inui, K. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J. Am. Soc. Nephrol. 2006, 17, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Terada, T.; Mizuno, T.; Katsura, T.; Shimakura, J.; Inui, K. Targeted disruption of the multidrug and toxin extrusion 1 (Mate1) gene in mice reduces renal secretion of metformin. Mol. Pharmacol. 2009, 75, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, A.; Inui, K. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br. J. Pharmacol. 2011, 164, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, M.; Masuda, S.; Watanabe, S.; Terada, T.; Katsura, T.; Inui, K. Renal tubular secretion of varenicline by multidrug and toxin extrusion (MATE) transporters. Drug Metab. Pharmacokinet. 2012, 27, 563–569. [Google Scholar] [CrossRef] [PubMed]
- EMA. Guideline on the Investigation of Drug Interactions. Availabler online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf (accessed on 20 May 2016).
- Hillgren, K.M.; Keppler, D.; Zur, A.A.; Giacomini, K.M.; Stieger, B.; Cass, C.E.; Zhang, L.; International Transporter Consortium. Emerging transporters of clinical importance: An update from the International Transporter Consortium. Clin. Pharmacol. Ther. 2013, 94, 52–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA. Guidance for Industry: Drug Interaction Studies—Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations (Draft Guidance). Availabler online: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm292362.pdf (accessed on 20 May 2016).
- Lipska, K.J.; Bailey, C.J.; Inzucchi, S.E. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care 2011, 34, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Minematsu, T.; Giacomini, K.M. Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Mol. Cancer Ther. 2011, 10, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Asaka, J.; Terada, T.; Tsuda, M.; Katsura, T.; Inui, K. Identification of essential histidine and cysteine residues of the H+/organic cation antiporter multidrug and toxin extrusion (MATE). Mol. Pharmacol. 2007, 71, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Mori, H.; Kito, T.; Yokochi, M.; Ito, S.; Inoue, K.; Yonezawa, A.; Katsura, T.; Kumagai, Y.; Yuasa, H.; et al. Investigation of endogenous compounds for assessing the drug interactions in the urinary excretion involving multidrug and toxin extrusion proteins. Pharm. Res. 2014, 31, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, Y.; Masuda, S.; Sato, T.; Katsura, T.; Ogawa, O.; Inui, K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H+-organic cation antiporters. Biochem. Pharmacol. 2007, 74, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cherrington, N.J.; Wright, S.H. Molecular identification and functional characterization of rabbit MATE1 and MATE2-K. Am. J. Physiol. Ren. Physiol. 2007, 293, F360–F370. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.S.; Satriano, J.A.; Pucci, M.; Schuster, V.L. Mechanism of prostaglandin E2 transport across the plasma membrane of HeLa cells and Xenopus oocytes expressing the prostaglandin transporter “PGT”. J. Biol. Chem. 1998, 273, 6689–6697. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Chan, B.S.; Schuster, V.L. Cloning of the human kidney PAH transporter: Narrow substrate specificity and regulation by protein kinase C. Am. J. Physiol. 1999, 276, F295–F303. [Google Scholar] [PubMed]
- Hegde, S.S.; Jadhav, A.L.; Lokhandwala, M.F. Role of kidney dopamine in the natriuretic response to volume expansion in rats. Hypertension 1989, 13, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.T.; Dimke, H.; Cordat, E. Proximal tubular NHEs: Sodium, protons and calcium? Am. J. Physiol. Ren. Physiol. 2013, 305, F229–F236. [Google Scholar] [CrossRef] [PubMed]
- Guilhot, F. Indications for imatinib mesylate therapy and clinical management. Oncologist 2004, 9, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Soares-Da-Silva, P.; Serrao, M.P.; Vieira-Coelho, M.A. Apical and basolateral uptake and intracellular fate of dopamine precursor l-DOPA in LLC-PK1 cells. Am. J. Physiol. 1998, 274, F243–F251. [Google Scholar] [PubMed]
- Nielsen, R.; Birn, H.; Moestrup, S.K.; Nielsen, M.; Verroust, P.; Christensen, E.I. Characterization of a kidney proximal tubule cell line, LLC-PK1, expressing endocytotic active megalin. J. Am. Soc. Nephrol. 1998, 9, 1767–1776. [Google Scholar] [PubMed]
- Pritchard, J.B.; Miller, D.S. Renal secretion of organic anions and cations. Kidney Int. 1996, 49, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Inui, K.; Masuda, S.; Saito, H. Cellular and molecular aspects of drug transport in the kidney. Kidney Int. 2000, 58, 944–958. [Google Scholar] [CrossRef] [PubMed]
- Maurel, A.; Spreux-Varoquaux, O.; Amenta, F.; Tayebati, S.K.; Tomassoni, D.; Seguelas, M.H.; Parini, A.; Pizzinat, N. Vesicular monoamine transporter 1 mediates dopamine secretion in rat proximal tubular cells. Am. J. Physiol. Ren. Physiol. 2007, 292, F1592–F1598. [Google Scholar] [CrossRef] [PubMed]
- Inui, K.; Saito, H.; Hori, R. H+-gradient-dependent active transport of tetraethylammonium cation in apical-membrane vesicles isolated from kidney epithelial cell line LLC-PK1. Biochem. J. 1985, 227, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, M.; Terada, T.; Ogasawara, K.; Iwano, J.; Katsura, T.; Fukatsu, A.; Doi, T.; Inui, K. Identification of multidrug and toxin extrusion (MATE1 and MATE2-K) variants with complete loss of transport activity. J. Hum. Genet. 2009, 54, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Toyama, K.; Yonezawa, A.; Tsuda, M.; Masuda, S.; Yano, I.; Terada, T.; Osawa, R.; Katsura, T.; Hosokawa, M.; Fujimoto, S.; et al. Heterozygous variants of multidrug and toxin extrusions (MATE1 and MATE2-K) have little influence on the disposition of metformin in diabetic patients. Pharmacogenet. Genom. 2010, 20, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Felder, C.C.; McKelvey, A.M.; Gitler, M.S.; Eisner, G.M.; Jose, P.A. Dopamine receptor subtypes in renal brush border and basolateral membranes. Kidney Int. 1989, 36, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Yao, B.; Wang, S.; Fan, X.; Wu, G.; Yang, H.; Yin, H.; Yang, S.; Harris, R.C. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J. Clin. Investig. 2011, 121, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Obach, R.S.; Burstein, A.H.; Clark, D.J.; de Morais, S.M.; Faessel, H.M. Effect of human renal cationic transporter inhibition on the pharmacokinetics of varenicline, a new therapy for smoking cessation: An in vitro-in vivo study. Clin. Pharmacol. Ther. 2008, 83, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [CrossRef] [PubMed]
- Gschwind, H.P.; Pfaar, U.; Waldmeier, F.; Zollinger, M.; Sayer, C.; Zbinden, P.; Hayes, M.; Pokorny, R.; Seiberling, M.; Ben-Am, M.; et al. Metabolism and disposition of imatinib mesylate in healthy volunteers. Drug Metab. Dispos. 2005, 33, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, R.L.; Buckle, T.; Beijnen, J.H.; van Tellingen, O.; Schellens, J.H. The effect of P-gp (Mdr1a/1b), BCRP (Bcrp1) and P-gp/BCRP inhibitors on the in vivo absorption, distribution, metabolism and excretion of imatinib. Investig. New Drugs 2009, 27, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, Y.; Masuda, S.; Katsura, T.; Inui, K. Protective effect of concomitant administration of imatinib on cisplatin-induced nephrotoxicity focusing on renal organic cation transporter OCT2. Biochem. Pharmacol. 2009, 78, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Steeghs, N.; Nijenhuis, C.M.; Schellens, J.H.; Beijnen, J.H.; Huitema, A.D. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: Focus on the pharmacokinetic targets. Clin. Pharmacokinet. 2014, 53, 305–325. [Google Scholar] [CrossRef] [PubMed]
- McClelland, C.M.; Harocopos, G.J.; Custer, P.L. Periorbital edema secondary to imatinib mesylate. Clin. Ophthalmol. 2010, 4, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.; Bazeos, A.; Mahon, F.X.; Eliasson, L.; Milojkovic, D.; Bua, M.; Apperley, J.F.; Szydlo, R.; Desai, R.; Kozlowski, K.; et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J. Clin. Oncol. 2010, 28, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Salaroli, A.; Serrao, A.; Alimena, G.; Breccia, M. Edema in patients treated with imatinib. J. Symptoms Signs 2013, 2, 78–83. [Google Scholar]
- Guan, Y.; Hao, C.; Cha, D.R.; Rao, R.; Lu, W.; Kohan, D.E.; Magnuson, M.A.; Redha, R.; Zhang, Y.; Breyer, M.D. Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nat. Med. 2005, 11, 861–866. [Google Scholar] [CrossRef] [PubMed]






| Kinetic Parameters | hMATE1 | hMATE2-K | mMATE1 |
|---|---|---|---|
| Km (mM) | 0.56 ± 0.18 * | 2.48 ± 0.65 ‡ | 0.53 ± 0.08 |
| Vmax (nmol·mg·protein−1·min−1) | 3.71 ± 0.15 * | 7.69 ± 1.12 | 8.73 ± 0.08 †† |
| Vmax/Km (µL·mg·protein−1·min−1) | 7.70 ± 1.67 | 3.44 ± 0.78 ‡‡ | 17.20 ± 2.72 † |
| Blood Parameters and Body Weight | Wild-Type Mice | Mate1-Knockout Mice |
|---|---|---|
| Na+ (mmol/L) | 147.0 ± 0.2 | 149.1 ± 0.6 ** |
| K+ (mmol/L) | 4.0 ± 0.1 | 3.8 ± 0.0 * |
| Cl− (mmol/L) | 113.6 ± 0.4 | 114.3 ± 0.7 |
| iCa (mmol/L) | 1.2 ± 0.0 | 1.2 ± 0.0 |
| tCO2 (mmol/L) | 19.3 ± 0.5 | 19.6 ± 0.4 |
| Glucose (mg/dL) | 249.9 ± 10.0 | 182.4 ± 17.3 ** |
| BUN (mg/dL) | 25.1 ± 1.1 | 26.0 ± 2.6 |
| Hct (%) | 38.9 ± 0.5 | 37.6 ± 2.1 |
| Hb (g/dL)(via Hct) | 13.2 ± 0.2 | 12.8 ± 0.7 |
| AnGap (mmol/L) | 19.1 ± 0.6 | 19.9 ± 0.8 |
| Body weight (g) | 28.7 ± 0.4 | 29.1 ± 0.4 |
| Blood Parameters and Body Weight | Vehicle-Treated Mice | Imatinib-Treated Mice |
|---|---|---|
| Na+ (mmol/L) | 147 ± 1.5 | 145.7 ± 1.5 |
| K+ (mmol/L) | 4.9 ± 0.3 | 4.5 ± 0.5 |
| Cl− (mmol/L) | 115.0 ± 0.6 | 115.3 ± 1.2 |
| iCa (mmol/L) | 1.2 ± 0.0 | 1.3 ± 0.0 |
| tCO2 (mmol/L) | 25.0 ± 0.6 | 19.7 ± 1.5 * |
| Glucose (mg/dL) | 284.0 ± 62.6 | 229.0 ± 39.3 |
| BUN (mg/dL) | 26.3 ± 0.3 | 21.0 ± 2.1 |
| Hct (%) | 39.7 ± 0.3 | 38.3 ± 2.0 |
| Hb (g/dL)(via Hct) | 13.5 ± 0.1 | 13.0 ± 0.7 |
| AnGap (mmol/L) | 13.0 ± 0.6 | 16.0 ± 1.5 |
| Body weight (g) | 26.6 ± 0.3 | 26.7 ± 0.8 |
| Transporter | Imatinib (µM) | Dasatinib (µM) | Nilotinib (µM) |
|---|---|---|---|
| hMATE1 | 1.1 ± 0.1 | 7.1 ± 0.6 | >100 |
| hMATE2-K | 13.8 ± 4.4 | 4.1 ± 1.0 | >100 |
| mMATE1 | 100.6 ± 13.9 | 106.2 ± 7.0 | >100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajiwara, M.; Ban, T.; Matsubara, K.; Nakanishi, Y.; Masuda, S. Urinary Dopamine as a Potential Index of the Transport Activity of Multidrug and Toxin Extrusion in the Kidney. Int. J. Mol. Sci. 2016, 17, 1228. https://doi.org/10.3390/ijms17081228
Kajiwara M, Ban T, Matsubara K, Nakanishi Y, Masuda S. Urinary Dopamine as a Potential Index of the Transport Activity of Multidrug and Toxin Extrusion in the Kidney. International Journal of Molecular Sciences. 2016; 17(8):1228. https://doi.org/10.3390/ijms17081228
Chicago/Turabian StyleKajiwara, Moto, Tsuyoshi Ban, Kazuo Matsubara, Yoichi Nakanishi, and Satohiro Masuda. 2016. "Urinary Dopamine as a Potential Index of the Transport Activity of Multidrug and Toxin Extrusion in the Kidney" International Journal of Molecular Sciences 17, no. 8: 1228. https://doi.org/10.3390/ijms17081228
APA StyleKajiwara, M., Ban, T., Matsubara, K., Nakanishi, Y., & Masuda, S. (2016). Urinary Dopamine as a Potential Index of the Transport Activity of Multidrug and Toxin Extrusion in the Kidney. International Journal of Molecular Sciences, 17(8), 1228. https://doi.org/10.3390/ijms17081228