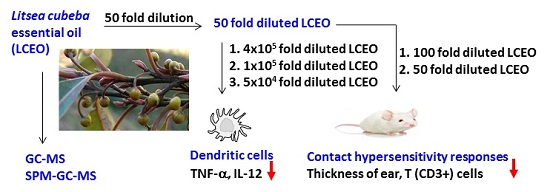
Immunosuppressive Effect of Litsea cubeba L. Essential Oil on Dendritic Cell and Contact Hypersensitivity Responses
Abstract
:1. Introduction
2. Results and Discussion
2.1. Constituents of the Essential Oils
2.2. L. cubeba Essential Oils (LCEO) Inhibited the Activation of Dendritic Cells (DCs)
2.3. The Contact Hypersensitivity (CHS) Response Is Attenuated in Mice Co-Treated with LCEO
3. Materials and Methods
3.1. Plant Material
3.2. Methods
3.2.1. Preparation of L. cubeba Essential Oil
3.2.2. Analysis of the Volatile Constituents
3.3. Preparation of Mouse Bone Marrow-Derived Dendritic Cells
3.4. Cytotoxicity Assay of LCEO
3.5. Measurement of Cytokines Production by DCs
3.6. The Assay of Contact Hypersensitivity (CHS) Response
3.7. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoid. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Guermonprez, P.; Valladeau, J.; Zitvogel, L.; Thery, C.; Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar] [CrossRef] [PubMed]
- Rudulier, C.D.; Kroeger, D.R.; Bretscher, P.A. Distinct roles of dendritic and B cells in the activation of naive CD4(+) T cells. Immunotherapy 2012, 4, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.K.; Yu, Y.L.; Chen, K.C.; Chang, W.T.; Lee, M.S.; Yang, M.J.; Cheng, H.C.; Liu, C.H.; Chen, D.C.; Chu, C.L. Kaempferol from Semen Cuscutae attenuates the immune function of dendritic cells. Immunobiology 2011, 216, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Pan, I.H.; Li, Y.R.; Pan, Y.G.; Lin, M.K.; Lu, Y.H.; Wu, H.C.; Chu, C.L. The adjuvant effects of high-molecule-weight polysaccharides purified from Antrodia cinnamomea on dendritic cell function and DNA vaccines. PLoS ONE 2015, 10, e0116191. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.K.; Lee, M.S.; Chang, W.T.; Chen, H.Y.; Chen, J.F.; Li, Y.R.; Lin, C.C.; Wu, T.S. Immunosuppressive effect of zhankuic acid C from Taiwanofungus camphoratus on dendritic cell activation and the contact hypersensitivity response. Bioorg. Med. Chem. Lett. 2015, 25, 4637–4641. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Yang, T.S. Antimicrobial impact of the components of essential oil of Litsea cubeba from Taiwan and antimicrobial activity of the oil in food systems. Int. J. Food Microbiol. 2012, 156, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Ou, J.P.; Liu, Y.C.; Hung, C.P.; Tsai, M.C.; Liao, P.C.; Wang, E.I.; Chen, Y.L.; Su, Y.C. Compositions and in vitro anticancer activities of the leaf and fruit oils of Litsea cubeba from Taiwan. Nat. Prod. Commun. 2010, 5, 617–620. [Google Scholar] [PubMed]
- Liao, P.C.; Yang, T.S.; Chou, J.C.; Chen, J.; Lee, S.C.; Kuo, Y.H.; Ho, C.L.; Chao, L.K.P. Anti-inflammatory activity of neral and geranial isolated from fruits of Litsea cubeba Lour. J. Funct. Foods 2015, 19, 248–258. [Google Scholar] [CrossRef]
- Jiang, Z.; Akhtar, Y.; Bradbury, R.; Zhang, X.; Isman, M.B. Comparative toxicity of essential oils of Litsea pungens and Litsea cubeba and blends of their major constituents against the cabbage looper, Trichoplusia ni. J. Agric. Food Chem. 2009, 57, 4833–4837. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y. Chemical composition and antibacterial activity of essential oils from different parts of Litsea cubeba. Chem. Biodivers. 2010, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.K.; Choi, E.M.; Lee, J.H. Antioxidant activity of Litsea cubeba. Fitoterapia 2005, 76, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Seal, S.; Priyajit Chatterjee, P.; Bhattacharya1, S.; Pal, D.; Dasgupta, S.; Kundu, R.; Mukherjee, S.; Bhattacharya, S.; Bhuyan, M.; Bhattacharyya, P.R.; et al. Vapor of volatile oils from Litsea cubeba seed induces apoptosis and causes cell cycle arrest in lung cancer cells. PLoS ONE 2012, 7, e47014. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.M.; Kim, J.; Lee, S.G.; Shin, C.H.; Sang-Chul Shin, S.C.; Park, I.K. Fumigant antitermitic activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica), caraway (Carum carvi), dill (Anethum graveolens), geranium (Pelargonium graveolens), and litsea (Litsea cubeba) oils against Japanese termite (Reticulitermes speratus Kolbe). J. Agric. Food Chem. 2009, 57, 6596–6602. [Google Scholar] [PubMed]
- Punyarajun, S.; Nandhasri, P. Volatile oil from Litsea cubeba in Thailand. Mahidol Univ. J. Pharm. Sci. 1981, 8, 65–71. [Google Scholar]
- Kejlová, K.; Jírová, D.; Bendová, H.; Gajdoš, P.; Kolářová, H. Phototoxicity of essential oils intended for cosmetic use. Toxicol. In Vitro 2010, 24, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiao, Y.; Liu, B.; Fang, X.; Yang, W.; Xu, J. Comparison of headspace solid-phase microextraction with conventional extraction for the analysis of the volatile components in Melia azedarach. Talanta 2011, 86, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, M.K.; He, R.; Kumar, L.; Yoon, J.; Geha, R.S. Cellular and molecular mechanisms in atopic dermatitis. Adv. Immunol. 2009, 102, 135–226. [Google Scholar] [PubMed]
- Schomburg, G.; Dielmann, G. Identification by means of retention parameters. J. Chromatogr. Sci. 1973, 11, 151–159. [Google Scholar] [CrossRef]
- Yeh, C.H.; Tsai, W.Y.; Chiang, H.M.; Wu, C.S.; Lee, Y.I.; Lin, L.Y.; Chen, H.C. Headspace solid-phase microextraction analysis of volatile components in Phalaenopsis Nobby’s Pacific Sunset. Molecules 2014, 19, 14080–14093. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Liao, N.S.; Lai, S.L.; Hsu, L.; Mao, W.Y.; Ku, M.C.; Liao, F. Reduced 2,4-dinitro-1-fluorobenzene–induced contact hypersensitivity response in IL-15 receptor a-deficient mice correlates with diminished CCL5/RANTES and CXCL10/IP-10 expression. Eur. J. Immunol. 2005, 35, 690–698. [Google Scholar] [CrossRef] [PubMed]




| Compound | RI z | Content (%) y | |
|---|---|---|---|
| DI/GC | HS-SPME/GC | ||
| Monoterpenes | 4.01 | 11.91 | |
| α-thujene | 921 | – w | 0.02 |
| α-pinene | 931 | 0.22 | 0.72 |
| camphene | 945 | 0.03 | 0.10 |
| sabinene | 972 | 0.07 | 0.06 |
| β-pinene | 976 | 0.09 | 0.20 |
| β-myrcene | 980 | 0.77 | 2.11 |
| α-phellandrene | 998 | – | 0.01 |
| α-terpinene | 1007 | <0.01 | – |
| ρ-cymene | 1014 | 0.01 | 0.01 |
| limonene | 1026 | 2.73 | 8.50 |
| cis-β-ocimene | 1026 | 0.01 | 0.02 |
| trans-β-ocimene | 1032 | – | 0.02 |
| γ-terpinene | 1050 | 0.01 | 0.01 |
| α-terpinolene | 1078 | 0.04 | 0.11 |
| 1,3,8-ρ-menthatriene | 1094 | 0.03 | 0.02 |
| Sesquiterpenes | 0.10 | 0.06 | |
| α-copaene | 1366 | 0.01 | 0.01 |
| β-elemene | 1382 | 0.04 | <0.01 |
| β-caryophyllene | 1429 | 0.03 | 0.04 |
| α-humulene | 1441 | 0.02 | 0.01 |
| δ-cadinene | 1525 | <0.01 | <0.01 |
| Terpene alcohols | 2.75 | 5.22 | |
| linalool | 1079 | 1.23 | 1.11 |
| isopulegol | 1128 | 0.03 | – |
| verbenol | 1130 | 1.31 | 3.81 |
| α-terpineol | 1183 | 0.07 | 0.06 |
| cis-carveol | 1189 | 0.07 | 0.19 |
| cis-geraniol | 1237 | 0.03 | 0.05 |
| nerolidol | 1558 | 0.01 | – |
| Terpene aldehydes | 89.25 | 75.09 | |
| citronellal | 1127 | 1.23 | 1.63 |
| neral | 1226 | 38.02 | 34.17 |
| geranial | 1256 | 50.00 | 39.29 |
| (2854.05 mmol/L) | |||
| Terpene ketone | 0.14 | 0.10 | |
| camphor | 1113 | 0.14 | 0.04 |
| piperitone | 1230 | <0.01 | <0.01 |
| piperitenone | 1308 | – | 0.06 |
| Terpene ester | 0.32 | 0.14 | |
| methyl salicylate | 1163 | 0.05 | 0.01 |
| bornyl acetate | 1286 | 0.01 | 0.02 |
| terpinenyl acetate | 1335 | 0.07 | 0.02 |
| citronellyl acetate | 1357 | 0.02 | 0.02 |
| geranyl acetate | 1362 | 0.16 | 0.06 |
| neryl acetate | 1366 | 0.01 | 0.01 |
| methyl cinnamate | 1384 | – | <0.01 |
| Terpene oxide | 0.16 | 0.17 | |
| 1,8-cineole | 1019 | 0.12 | 0.14 |
| trans-linalool oxide | 1055 | <0.01 | 0.02 |
| cis-rose oxide | 1086 | <0.01 | <0.01 |
| trans-rose oxide | 1089 | – | <0.01 |
| limonene oxide | 1128 | 0.01 | – |
| caryophyllene oxide | 1571 | 0.03 | 0.01 |
| Aliphatic aldehydes | 0.01 | 0.03 | |
| 3-methyl butanal | 631 | <0.01 | – |
| 2-methyl butanal | 636 | <0.01 | – |
| pentanal | 697 | – | <0.01 |
| hexanal | 776 | <0.01 | 0.01 |
| 2,6-dimethyl hept-5-enal | 1047 | 0.01 | 0.02 |
| Aliphatic ketone | 1.19 | 2.23 | |
| 6-methyl-5-hepten-2-one | 962 | 1.19 | 2.23 |
| Aliphatic alcohol | – | <0.01 | |
| 2-methyl-3-buten-2-ol | 600 | – | <0.01 |
| Aliphatic esters | 0.01 | 0.01 | |
| ethyl isovalerate | 825 | <0.01 | – |
| isoamyl acetate | 864 | 0.01 | 0.01 |
| ethyl tiglate | 915 | <0.01 | <0.01 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-C.; Chang, W.-T.; Hseu, Y.-C.; Chen, H.-Y.; Chuang, C.H.; Lin, C.-C.; Lee, M.-S.; Lin, M.-K. Immunosuppressive Effect of Litsea cubeba L. Essential Oil on Dendritic Cell and Contact Hypersensitivity Responses. Int. J. Mol. Sci. 2016, 17, 1319. https://doi.org/10.3390/ijms17081319
Chen H-C, Chang W-T, Hseu Y-C, Chen H-Y, Chuang CH, Lin C-C, Lee M-S, Lin M-K. Immunosuppressive Effect of Litsea cubeba L. Essential Oil on Dendritic Cell and Contact Hypersensitivity Responses. International Journal of Molecular Sciences. 2016; 17(8):1319. https://doi.org/10.3390/ijms17081319
Chicago/Turabian StyleChen, Hsin-Chun, Wen-Te Chang, You-Cheng Hseu, Hsing-Yu Chen, Cheng Hsuan Chuang, Chi-Chen Lin, Meng-Shiou Lee, and Ming-Kuem Lin. 2016. "Immunosuppressive Effect of Litsea cubeba L. Essential Oil on Dendritic Cell and Contact Hypersensitivity Responses" International Journal of Molecular Sciences 17, no. 8: 1319. https://doi.org/10.3390/ijms17081319
APA StyleChen, H. -C., Chang, W. -T., Hseu, Y. -C., Chen, H. -Y., Chuang, C. H., Lin, C. -C., Lee, M. -S., & Lin, M. -K. (2016). Immunosuppressive Effect of Litsea cubeba L. Essential Oil on Dendritic Cell and Contact Hypersensitivity Responses. International Journal of Molecular Sciences, 17(8), 1319. https://doi.org/10.3390/ijms17081319