Flavonoids Extracted from Licorice Prevents Colitis-Associated Carcinogenesis in AOM/DSS Mouse Model
Abstract
:1. Introduction
2. Results
2.1. LFs Increased the Survival Rate of AOM/DSS Induced Mice
2.2. LFs Suppresses Colitis-Associated Colon Tumorigenesis
2.3. LFs Regulated Proteins Associated with Apoptosis and Proliferation in Colonic Tissues
2.4. LFs Treatment Reduced Pro-Inflammatory Cytokines and Mediators
2.5. LFs Treatment Attenuated Phosphorylation of Jak2 and Stat3
2.6. LFs Inhibited Activation of Nfκb
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
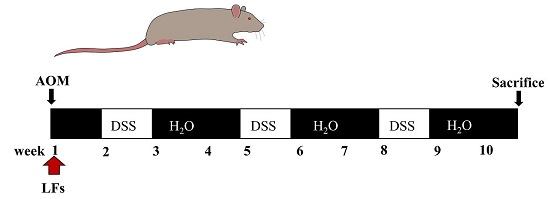
4.3. AOM/DSS-Induced Colitis-Associated Carcinogenesis
4.4. Histopathological Analysis
4.5. Immunohistochemical (IHC) Analysis
4.6. Measurement of Pro-Inflammatory Cytokines (IL-1β, IL-6, and TNF-α)
4.7. RNA Isolation and Quantitative Real Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sobczak, M.; Wlazłowski, M.; Zatorski, H.; Salaga, M.; Fichna, J. Current overview of colitis-associated colorectal cancer. Cent. Eur. J. Biol. 2014, 9, 1022–1029. [Google Scholar] [CrossRef]
- Moriasi, C.; Subramaniam, D.; Awasthi, S.; Ramalingam, S.; Anant, S. Prevention of colitis-associated cancer: Natural compounds that target the IL-6 soluble receptor. Anticancer Agents Med. Chem. 2012, 12, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Chumanevich, A.A.; Poudyal, D.; Cui, X.; Davis, T.; Wood, P.A.; Smith, C.D.; Hofseth, L.J. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis 2010, 31, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Kathiria, A.S.; Neumann, W.L.; Rhees, J.; Hotchkiss, E.; Cheng, Y.; Genta, R.M.; Meltzer, S.J.; Souza, R.F.; Theiss, A.L. Prohibitin attenuates colitis-associated tumorigenesis in mice by modulating P53 and Stat3 apoptotic responses. Cancer Res. 2012, 72, 5778–5789. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. Interleukin-6: From basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Garcia Rodriguez, L.A.; Cea-Soriano, L.; Tacconelli, S.; Patrignani, P. Coxibs: Pharmacology, toxicity and efficacy in cancer clinical trials. Recent Results Cancer Res. 2013, 191, 67–93. [Google Scholar] [PubMed]
- Tang, Z.H.; Li, T.; Tong, Y.G.; Chen, X.J.; Chen, X.P.; Wang, Y.T.; Lu, J.J. A systematic review of the anticancer properties of compounds isolated from licorice (gancao). Planta Med. 2015, 81, 1670–1687. [Google Scholar] [CrossRef] [PubMed]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, X.; Chen, X.; Li, Y.; Ke, Z.; Tang, T.; Chai, H.; Guo, A.M.; Chen, H.; Yang, J. Isoliquiritigenin, a flavonoid from licorice, blocks M2 macrophage polarization in colitis-associated tumorigenesis through downregulating PGE2 and IL-6. Toxicol. Appl. Pharmacol. 2014, 279, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Li, H.; Wang, X.; Lee, F.S.; Cui, S. Isolation and identification of flavonoids in licorice and a study of their inhibitory effects on tyrosinase. J. Agric. Food Chem. 2005, 53, 7408–7414. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, B.; Mensinga, T.; Sips, A.; Seinen, W.; Meulenbelt, J.; DeJongh, J. The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling. Drug Metab. Rev. 2001, 33, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Park, K.K.; Lim, S.S.; Park, J.H.; Chung, W.Y. Effects of the licorice extract against tumor growth and cisplatin-induced toxicity in a mouse xenograft model of colon cancer. Biol. Pharm Bull. 2007, 30, 2191–2195. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.C.; Dong, X.W.; Wu, X.M.; Yan, X.F.; Xie, Q.M. Inhibitory effects of flavonoids extracted from licorice on lipopolysaccharide-induced acute pulmonary inflammation in mice. Int. Immunopharmacol. 2009, 9, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Dongyu, L.; Li, G.; Juan, Z.; Xiaowei, H.; Xiaoling, M.; Jianyong, S.; Hui, N.; Li, C. Anti-inflammatory and antioxidant effects of licorice flavonoids on ulcerative colitis in mouse models. Int. Immunopharmacol. 2016. major revisions. [Google Scholar]
- Neumann, H.; Vieth, M.; Langner, C.; Neurath, M.F.; Mudter, J. Cancer risk in IBD: How to diagnose and how to manage dalm and alm. World J. Gastroenterol. 2011, 17, 3184–3191. [Google Scholar] [PubMed]
- Madka, V.; Rao, C.V. Anti-inflammatory phytochemicals for chemoprevention of colon cancer. Curr. Cancer Drug Targets 2013, 13, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.D.; Wang, C.Z.; Yu, C.; Zhao, L.; Zhang, Z.; Matin, A.; Wang, Y.; Li, P.; Xiao, S.Y.; Du, W.; et al. Panax notoginseng attenuates experimental colitis in the azoxymethane/dextran sulfate sodium mouse model. Phytother. Res. 2014, 28, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Neufert, C.; Becker, C.; Neurath, M.F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2007, 2, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kargl, J.; Haybaeck, J.; Stancic, A.; Andersen, L.; Marsche, G.; Heinemann, A.; Schicho, R. O-1602, an atypical cannabinoid, inhibits tumor growth in colitis-associated colon cancer through multiple mechanisms. J. Mol. Med. (Berl.) 2013, 91, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Coudry, R.A.; Clapper, M.L.; Zhang, X.; Williams, K.L.; Spittle, C.S.; Li, T.; Cooper, H.S. Loss of P53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis 2007, 28, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Jeppsson, S.; Dalmasso, G.; Ghaleb, A.M.; McConnell, B.B.; Yang, V.W.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Notch1 regulates the effects of matrix metalloproteinase-9 on colitis-associated cancer in mice. Gastroenterology 2011, 141, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. P53, the cellular gatekeeper for growth and division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef]
- Cory, S.; Huang, D.C.; Adams, J.M. The BcI-2 family: Roles in cell survival and oncogenesis. Oncogene 2003, 22, 8590–8607. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Neurath, M.F. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin. Rev. Allergy Immunol. 2005, 28, 187–196. [Google Scholar] [CrossRef]
- Klampfer, L. The role of signal transducers and activators of transcription in colon cancer. Front. Biosci. 2008, 13, 2888–2899. [Google Scholar] [CrossRef] [PubMed]
- Bollrath, J.; Greten, F.R. IKK/NFκB and Stat3 pathways: Central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009, 10, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Neurath, M.F. Signaling molecules: The pathogenic role of the IL-6/Stat3 trans signaling pathway in intestinal inflammation and in colonic cancer. Curr. Drug Targets 2008, 9, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yi, Z.; Diao, J.; Shao, M.; Zhao, L.; Cai, H.; Fan, Q.; Yao, X.; Sun, X. Shaoyao decoction ameliorates colitis-associated colorectal cancer by downregulating proinflammatory cytokines and promoting epithelial-mesenchymal transition. J. Transl. Med. 2014, 12, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.C.; Wilson, J.E.; Schneider, M.; Lich, J.D.; Roberts, R.A.; Arthur, J.C.; Woodford, R.M.; Davis, B.K.; Uronis, J.M.; Herfarth, H.H.; et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NFκB signaling. Immunity 2012, 36, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, W.K.; Li, Z.J.; Chan, K.M.; Wong, C.C.; Ye, C.G.; Yu, L.; Sung, J.J.; Cho, C.H.; Wang, M. 2,3′,4,4′,5′-pentamethoxy-trans-stilbene, a resveratrol derivative, inhibits colitis-associated colorectal carcinogenesis in mice. Br. J. Pharmacol. 2010, 160, 1352–1361. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, X.; Liu, D.; Gao, L.; Li, L.; Cao, L. Flavonoids Extracted from Licorice Prevents Colitis-Associated Carcinogenesis in AOM/DSS Mouse Model. Int. J. Mol. Sci. 2016, 17, 1343. https://doi.org/10.3390/ijms17091343
Huo X, Liu D, Gao L, Li L, Cao L. Flavonoids Extracted from Licorice Prevents Colitis-Associated Carcinogenesis in AOM/DSS Mouse Model. International Journal of Molecular Sciences. 2016; 17(9):1343. https://doi.org/10.3390/ijms17091343
Chicago/Turabian StyleHuo, Xiaowei, Dongyu Liu, Li Gao, Liyong Li, and Li Cao. 2016. "Flavonoids Extracted from Licorice Prevents Colitis-Associated Carcinogenesis in AOM/DSS Mouse Model" International Journal of Molecular Sciences 17, no. 9: 1343. https://doi.org/10.3390/ijms17091343
APA StyleHuo, X., Liu, D., Gao, L., Li, L., & Cao, L. (2016). Flavonoids Extracted from Licorice Prevents Colitis-Associated Carcinogenesis in AOM/DSS Mouse Model. International Journal of Molecular Sciences, 17(9), 1343. https://doi.org/10.3390/ijms17091343