Plant Polyphenols as Chemopreventive Agents for Lung Cancer
Abstract
:1. Introduction
2. Nature of Polyphenols and Their Classification
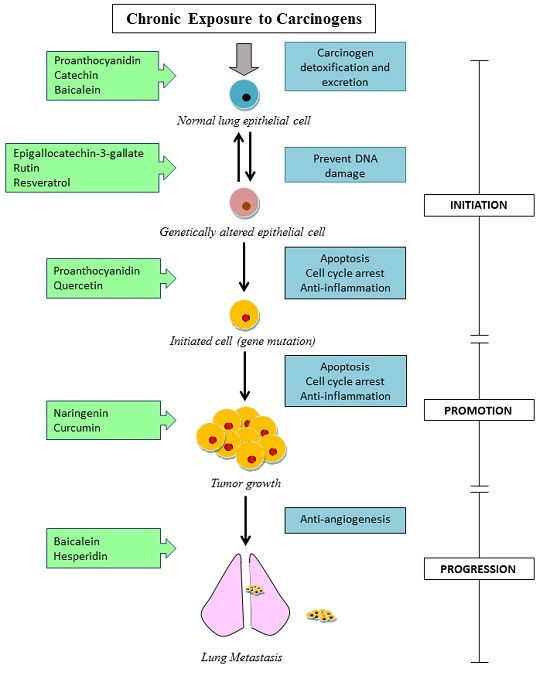
3. Characteristics of Polyphenols in Cancer Prevention
4. Environmental and Occupation Lung Carcinogens
- Group 1: Carcinogenic to human.
- Group 2A: Probably carcinogenic to human.
- Group 2B: Possibly carcinogenic to human.
- Group 3: Not classifiable as it’s carcinogenic to human.
- Group 4: Probably not carcinogenic to humans.
5. Evidences for Lung Cancer Prevention by Dietary Polyphenols
5.1. In Vitro Studies
5.2. In Vivo Studies
6. Epidemiological Evidence of Lung Cancer Prevention
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- The American Cancer Society & LiveStrong. The Global Economic Cost of Cancer. Available online: http://www.cancer.org/acs/groups/content/@internationalaffairs/documents/document/acspc-026203.pdf (accessed on 5 July 2016).
- Morgan, E. Cancer Control: Knowledge into Action: WHO Guide for Effective Programmes; WHO: Geneva, Switzerland, 2007; Volume 2, pp. 1–49. [Google Scholar]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Joint WHO; FAO Expert Consultation. Diet, Nutrition and the Prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003, 916, 1–8. [Google Scholar]
- Buchner, F.L.; Bueno-de-Mesquita, H.B.; Linseisen, J.; Boshuizen, H.C.; Kiemeney, L.A.; Ros, M.M.; Overvad, K.; Hansen, L.; Tjonneland, A.; Raaschou-Nielsen, O.; et al. Fruits and vegetables consumption and the risk of histological subtypes of lung cancer in the European prospective investigation into cancer and nutrition (EPIC). Cancer Causes Control. 2010, 21, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Linseisen, J.; Rohrmann, S.; Miller, A.B.; Bueno-De-Mesquita, H.; Buchner, F.L.; Vineis, P.; Agudo, A.; Gram, I.T.; Janson, L.; Krogh, V.; et al. Fruit and vegetable consumption and lung cancer risk: Updated information from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int. J. Cancer 2007, 121, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Nair, S.V.G.; Robinson, R.A. Chemopreventive properties of fruit phenolic compounds and their possible mode of actions. Stud. Nat. Prod. Chem. 2014, 42, 229–266. [Google Scholar]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and Western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Isoflavone Content of Selected Foods. Available online: http://www.ars.usda.gov/News/docs.htm?docid=6382 (accessed on 5 July 2016).
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Flavonoid Content of U.S. Fruits, Vegetables, and Nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; Taylor & Francis: Boca Raton, FL, USA, 2004. [Google Scholar]
- Spencer, J.P.E.; Abd El Mohsen, M.M.; Minihane, A.M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2008, 99, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhao, B.; Li, M.; Shen, S.; Xin, W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta-Lipids Lipid Metab. 1996, 1304, 210–222. [Google Scholar] [CrossRef]
- Denison, M.S.; Pandini, A.; Nagy, S.R.; Baldwin, E.P.; Bonati, L. Ligand binding and activation of the Ah receptor. Chem. Biol. Interact. 2002, 141, 3–24. [Google Scholar] [CrossRef]
- Denison, M.S.; Nagy, S.R. Activation of the arylhydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Hydroxyl radical and hypochlorous acid scavenging activity of small centaury (Centaurium erythraea) infusion. A comparative study with green tea (Camellia sinensis). Phytomedicine 2003, 10, 517–522. [Google Scholar]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden, B. Dirk Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II drug metabolizing enzymes. Biomed. Pap. 2010, 154, 103–116. [Google Scholar] [CrossRef]
- Zhai, X.; Lin, M.; Zhang, F.; Hu, Y.; Xu, X.; Li, Y.; Liu, K.; Ma, X.; Tian, X.; Yao, J. Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells. Mol. Nutr. Food Res. 2013, 57, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H. Mechanisms underlying chemopreventive effects of flavonoids via multiple signaling nodes within Nrf2-ARE and AhR-XRE gene regulatory networks. Curr. Chem. Biol. 2013, 7, 151–176. [Google Scholar] [CrossRef]
- Klaassen, C.D. Casarett and Doull’s Toxicology: The Basic Science of Poisons; McGraw-Hill Education: New York, NY, USA, 2001; pp. 15–1454. [Google Scholar]
- Aoki, Y.; Sato, H.; Nishimura, N.; Takahashi, S.; Itoh, K.; Yamamoto, M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 2001, 173, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Hashimoto, A.H.; Amanuma, K.; Matsumoto, M.; Hiyoshi, K.; Takano, H.; Masumura, K.I.; Itoh, K.; Nohmi, T.; Yamamoto, M. Enhanced spontaneous and benzo(a)pyrene-induced mutations in the lung of Nrf2-deficient gpt delta mice. Cancer Res. 2007, 67, 5643–5648. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Jayvinen, R.; Seppsnen, R.; Helibvaara, M.; Teppo, L.; Pukkala, E. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997, 146, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Le Marchand, L.; Murphy, S.P.; Hankin, J.H.; Wilkens, L.R.; Kolonel, L.N. Intake of flavonoids and lung cancer. J. Natl. Cancer Inst. 2000, 92, 154–160. [Google Scholar] [CrossRef]
- Russo, G.L. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem. Pharmacol. 2007, 74, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.Y.; Naidu, A.; Parent, M.; Pintos, J.; Abrahamowicz, M.; Siemiatycki, J.; Koushik, A. The risk of lung cancer related to dietary intake of flavonoids. Nutr. Cancer 2012, 64, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Vaid, M.; Katiyar, S.K. Grape seed proanthocyanidins inhibit cigarette smoke condensate-induced lung cancer cell migration through inhibition of NADPH oxidase and reduction in the binding of p22phox and p47phox proteins. Mol. Carcinog. 2015, 54, E61–E71. [Google Scholar] [CrossRef] [PubMed]
- Punathil, T.; Katiyar, S.K. Inhibition of non-small cell lung cancer cell migration by grape seed proanthocyanidins is mediated through the inhibition of nitric oxide, guanylate cyclase, and ERK1/2. Mol. Carcinog. 2009, 48, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Sharma, S.D.; Katiyar, S.K. Grape proanthocyanidins induce apoptosis by loss of mitochondrial membrane potential of human non-small cell lung cancer cells in vitro and in vivo. PLoS ONE 2011, 6, e27444. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hsia, T.; Kuo, H.; Chao, P.L.; Chou, C.; Wei, Y.; Chung, J. Inhibition of lung cancer cell growth by quercetin glucuronides via G 2/M arrest and induction of apoptosis. Drug Metab. Dispos. 2006, 34, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, L.; Wang, H.; Ma, J.; Sun, J. Curcumin inhibits lung cancer progression and metastasis through induction of FOXO1. Tumour Biol. 2014, 35, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Kuzuhara, T.; Echigo, N.; Fujii, A.; Suganuma, M.; Fujiki, H. Apoptosis of human lung cancer cells by curcumin mediated through up-regulation of “growth arrest and DNA damage inducible genes 45 and 153”. Biol. Pharm. Bull. 2010, 33, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, C.; Jung, E.S.; Kim, J.S.; Kim, E. Antimetastatic activity of polyphenol-rich extract of Ecklonia cava through the inhibition of the Akt pathway in A549 human lung cancer cells. Food Chem. 2011, 127, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Heymach, J.V.; Lippman, S.M. Lung cancer. N. Engl. J. Med. 2008, 359, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Franklin, W.; Noguchi, M.; Gonzales, A. Molecular and cellular pathology of lung cancer. Princ. Pract. Lung Cancer 2010, 77, 287–324. [Google Scholar]
- Hanahan, D.; Weinberg, R.A.; Francisco, S. The hallmarks of cancer review. University of California at San Francisco. Horm. Res. 2000, 100, 57–70. [Google Scholar]
- Greenlee, R.T.; Murray, T.; Bolden, S.; Wingo, P.A. Cancer statistics, 2000. CA Cancer J. Clin. 2000, 50, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Müller-hermelink, H.K.; Harris, C.C. Pathology & genetics, tumours of the lung, pleura, thymus and heart. In World Health Organization Classification of Tumors 2004; IARC Press: Lyon, France, 2004; Volume 1, pp. 9–123. [Google Scholar]
- Yakut, T.; Schulten, H.J.; Demir, A.; Frank, D.; Danner, B.; Egeli, Ü.; Gebitekin, C.; Kahler, E.; Gunawan, B.; Ürer, N.; et al. Assessment of molecular events in squamous and non-squamous cell lung carcinoma. Lung Cancer 2006, 54, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Fontecedro, A.C.; Stahel, R.A.; Peters, S. Lung cancer. In Side Effects of Medical Cancer Therapy; Dicato, M.A., Ed.; Springer-Verlag: London, UK, 2013; pp. 119–138. [Google Scholar]
- International Agency for Research on Cancer. Occupational exposures during Aluminium production. IARC Monogr. 2010, 2005, 215–224. [Google Scholar]
- International Agency for Research on Cancer. Asenic and arcenic Compounds. IARC Monogr. 2004, 2002, 41–93. [Google Scholar]
- Ding, X.; Boney-Montoya, J.; Owen, B.M.; Bookout, A.L.; Mangelsdorf, D.J.; Kliewer, S.A. Occupational and environmental causes of lung cancer. Clin. Chest Med. 2013, 16, 387–393. [Google Scholar]
- International Agency for Research on Cancer. Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). IARC Monogr. Eval. Carcinog. Risks Hum. 2011, 100C, 219–309. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 92, 1–853. [Google Scholar]
- International Agency for Research on Cancer. Beryllium and beryllium compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 2011, 100C, 95–120. [Google Scholar]
- International Agency for Research on Cancer. Cadmium and cadmium compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 2011, 100C, 121–145. [Google Scholar]
- International Agency for Research on Cancer. Bis (chloromethyl) ether and chloromethyl methyl ether. World Heal. Organ. 2009, 1987, 295–308. [Google Scholar]
- International Agency for Research on Cancer. Chromium (VI) compounds. IARC Monogr. Arsen. Met. Fibres, Dusts Rev. Hum. Carcinog. 2012, 1989, 147–168. [Google Scholar]
- International Agency for Research on Cancer. Silica dust, crystalline, in the form of quartz or cristobalite. IARC Monogr. Eval. Carcinog. Risks Hum. 2011, 100C, 355–405. [Google Scholar]
- International Agency for Research on Cancer. Coal gasification. Iarc Monogr. Eval. Carcinog. Risks Hum. 2012, 100F, 145–152. [Google Scholar]
- International Agency for Research on Cancer. Coke production. Ap 42 1995, 2005, 167–178. [Google Scholar]
- International Agency for Research on Cancer. Summary of data reported and evaluation. World Heal. Organ. 2011, 105, 451–467. [Google Scholar]
- International Agency for Research on Cancer. Indoor emissions from household combustion of coal. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100E, 519–542. [Google Scholar]
- International Agency for Research on Cancer. Occupational exposures during iron and steel founding. World Heal. Organ. 1987, 1987, 497–508. [Google Scholar]
- International Agency for Research on Cancer. MOPP. World Heal. Organ. 2008, 1987, 63–92. [Google Scholar]
- International Agency for Research on Cancer. Nickel and nickel compounds. IARC Monogr. Eval. Carcinog. Risks Hum. 2011, 100C, 169–218. [Google Scholar]
- International Agency for Research on Cancer. N -nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100E, 323–335. [Google Scholar]
- International Agency for Research on Cancer. Occupational exposure as a painter. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100F, 509–539. [Google Scholar]
- International Agency for Research on Cancer. Internalized α-particle emitting. World Heal. Organ. 2001, 2000, 241–283. [Google Scholar]
- International Agency for Research on Cancer. Occupational exposures in the rubber-manufacturing industry. World Heal. Organ. 1987, 100F, 541–562. [Google Scholar]
- Öberg, M.; Woodward, A.; Jaakkola, M.S.; Peruga, A.; Prüss-Ustün, A. Global estimate of the burden of disease from second-hand smoke. World Heal. Organ. 2010, 1–81. [Google Scholar]
- International Agency for Research on Cancer. Second-hand tobacco smoke. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100E, 215–265. [Google Scholar]
- International Agency for Research on Cancer. Soot, as found in occupational exposure of chimney sweeps. World Heal. Organ. 2010, 1987, 209–214. [Google Scholar]
- International Agency for Research on Cancer. Sulfur mustard. World Heal. Organ. 2006, 1987, 437–450. [Google Scholar]
- Cooper, D.N. The Molecular Genetics of Lung Cancer; Springer-Verlag: Berlin, Germany, 2005. [Google Scholar]
- National Research Council. Health Effects of Exposure to Radon; National Academies Press: Washington, DC, USA, 1999. [Google Scholar]
- Soria, J.; Kim, E.S.; Fayette, J.; Lantuejoul, S.; Deutsch, E.; Hong, W.K. Reviews chemoprevention of lung cancer. Lancet Oncol. 2003, 4, 659–669. [Google Scholar] [CrossRef]
- Giudice, A.; Montella, M. Activation of the Nrf2-ARE signaling pathway: A promising strategy in cancer prevention. BioEssays 2006, 28, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Reuland, D.J.; Khademi, S.; Castle, C.J.; Irwin, D.C.; McCord, J.M.; Miller, B.F.; Hamilton, K.L. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic. Biol. Med. 2013, 56, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Konczak, I.; Sze, D.M.Y.; Ramzan, I. Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr. Cancer 2011, 63, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Sun, H.; Kluz, T.; Clancy, H.A.; Kiok, K.; Costa, M. Epigallocatechin-3-gallate (EGCG) protects against chromate-induced toxicity in vitro. Toxicol. Appl. Pharmacol. 2012, 258, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Zhong, Z.F.; Chen, M.W.; Bao, J.L.; Wu, G.S.; Zhang, Q.W.; Lee, S.M.Y.; Hoi, P.M.; Wang, Y.T. Bisdemethoxycurcumin increases Sirt1 to antagonize t-BHP-induced premature senescence in WI38 fibroblast cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 851714. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Lee, K.H.; Zhang, R.; Piao, M.J.; Chae, S.; Kim, K.N.; Jeon, Y.J.; Park, D.B.; You, H.J.; Kim, J.S.; et al. Caffeic acid protects hydrogen peroxide induced cell damage in WI-38 human lung fibroblast cells. Biol. Pharm. Bull. 2006, 29, 1820–1824. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, P.A.; Walle, T. Inhibition of benzo[a]pyrene-activating enzymes and DNA binding in human bronchial epithelial BEAS-2B cells by methoxylated flavonoids. Carcinogenesis 2006, 27, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Song, J.H.; Oh, M.H.; Lee, Y.J.; Kim, Y.B.; Im, J.H.; Lee, S.H. ERK1/2 activation in quercetin-treated BEAS-2B cell plays a role in Nrf2-driven HO-1 expression. Mol. Cell. Toxicol. 2011, 7, 347–355. [Google Scholar] [CrossRef]
- Tan, X.; Jin, P.; Feng, L.; Song, J.; Sun, E.; Liu, W.; Shu, L.; Jia, X. Protective effect of luteolin on cigarette smoke extract-induced cellular toxicity and apoptosis in normal human bronchial epithelial cells via the Nrf2 pathway. Oncol. Rep. 2014, 31, 1855–1862. [Google Scholar] [PubMed]
- Puppala, D.; Gairola, C.G.; Swanson, H.I. Identification of kaempferol as an inhibitor of cigarette smoke-induced activation of the aryl hydrocarbon receptor and cell transformation. Carcinogenesis 2007, 28, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Ekambaram, G.; Sakthisekaran, D. Cytoprotective effect of mangiferin on benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Basic Clin. Pharmacol. Toxicol. 2008, 103, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Balansky, R.; Ganchev, G.; Iltcheva, M.; Kratchanova, M.; Denev, P.; Kratchanov, C.; Polasa, K.; D’Agostini, F.; Steele, V.E.; de Flora, S. Inhibition of lung tumor development by berry extracts in mice exposed to cigarette smoke. Int. J. Cancer 2012, 131, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, S.; Anandakumar, P.; Jagan, S.; Ramakrishnan, G.; Devaki, T. Modulatory effect of hesperidin on benzo(a)pyrene induced experimental lung carcinogenesis with reference to COX-2, MMP-2 and MMP-9. Eur. J. Pharmacol. 2010, 649, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Naveenkumar, C.; Raghunandhakumar, S.; Asokkumar, S.; Binuclara, J.; Devaki, T. Baicalein improves antioxidant status and membrane-bound enzymes during oxidative stress in benzo(a)pyrene-induced lung carcinogenesis in mice. Biomed. Prev. Nutr. 2012, 2, 138–144. [Google Scholar] [CrossRef]
- Naveenkumar, C.; Raghunandakumar, S.; Asokkumar, S.; Binuclara, J.; Rajan, B.; Premkumar, T.; Devaki, T. Mitigating role of baicalein on lysosomal enzymes and xenobiotic metabolizing enzyme status during lung carcinogenesis of Swiss albino mice induced by benzo(a)pyrene. Fundam. Clin. Pharmacol. 2014, 28, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.J.; Shen, J.; Jia, Y.L.; Li, F.F.; Ma, W.J.; Shen, H.J.; Shen, L.L.; Lin, X.X.; Zhang, L.H.; Dong, X.W.; et al. Apple polyphenol protects against cigarette smoke-induced acute lung injury. Nutrition 2013, 29, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Bodduluru, L.N.; Kasala, E.R.; Madhana, R.M.; Barua, C.C.; Hussain, M.I.; Haloi, P.; Borah, P. Naringenin ameliorates inflammation and cell proliferation in benzo(a)pyrene induced pulmonary carcinogenesis by modulating CYP1A1, NFκB and PCNA expression. Int. Immunopharmacol. 2016, 30, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, N.; Suresh, G.; Ramesh, B.; Vijaiyan, S.G. Fisetin, a novel flavonol attenuates benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Food Chem. Toxicol. 2011, 49, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Chen, J.X.; Wang, H.; Lu, G.; Liu, A.; Li, G.; Tu, S.; Lin, Y.; Yang, C.S. NNK-induced DNA methyltransferase 1 in lung tumorigenesis in A/J mice and inhibitory effects of (−)-epigallocatechin-3-gallate. Nutr. Cancer 2015, 67, 167–176. [Google Scholar] [CrossRef] [PubMed]
- El-Kenawy, A.E.; Elshama, S.S.; Osman, H.H. Effects of Physalis peruviana L. on toxicity and lung cancer induction by nicotine derived nitrosamine ketone in rats. Asian Pac. J. Cancer Prev. 2015, 16, 5863–5868. [Google Scholar] [CrossRef]
- Chan, S.T.; Chuang, C.H.; Yeh, C.L.; Liao, J.W.; Liu, K.L.; Tseng, M.J.; Yeh, S.L. Quercetin supplementation suppresses the secretion of pro-inflammatory cytokines in the lungs of Mongolian gerbils and in A549 cells exposed to benzo[a]pyrene alone or in combination with β-carotene: In vivo and ex vivo studies. J. Nutr. Biochem. 2012, 23, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Patterson, R.E.; Thornquist, M.D.; Caret, T.; Omenn, G.S.; King, I.B.; Goodman, G.E. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the β-carotene and retinol efficacy trial (CARET). Cancer Epidemiol. Biomark. Prev. 2003, 12, 350–358. [Google Scholar]
- Zhong, L.; Goldberg, M.S.; Gao, Y.; Hanley, J.A.; Parent, M.; Jin, F. A Population-based case-control study of lung cancer and green tea consumption among women living in Shanghai, China. Epidemiology 2001, 12, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Ziegler, R.G.; Michaud, D.S.; Giovannucci, E.L.; Speizer, F.E.; Willett, W.C.; Colditz, G. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J. Natl. Cancer Inst. 2000, 92, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Rachtan, J.; Sokolowski, A. Risk factors for lung cancer among women in Poland. Lung Cancer 1997, 18, 137–145. [Google Scholar] [CrossRef]
- Yuan, J.; Stram, D.O.; Arakawa, K.; Lee, H.; Yu, M.C. Dietary cryptoxanthin and reduced risk of lung cancer: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2003, 12, 890–898. [Google Scholar]
- Männistö, S.; Smith-Warner, S.A.; Spiegelman, D.; Albanes, D.; Anderson, K.; van Den Brandt, P.A.; Cerhan, J.R.; Colditz, G.; Feskanich, D.; Freudenheim, J.L.; et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol. Biomark. Prev. 2004, 13, 40–48. [Google Scholar] [CrossRef]
- Büchner, F.L.; Bueno-de-Mesquita, H.B.; Ros, M.M.; Overvad, K.; Dahm, C.C.; Hansen, L.; Tjønneland, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Touillaud, M.; et al. Variety in fruit and vegetable consumption and the risk of lung cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Holick, C.N.; Michaud, D.S.; Stolzenberg-Solomon, R.; Mayne, S.T.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Dietary carotenoids, serumβ-carotene, and retinol and risk of lung cancer in the α-tocopherol, β-carotene cohort study. Am. J. Epidemiol. 2002, 156, 536–547. [Google Scholar] [CrossRef] [PubMed]



| Cell Type | Polyphenol | Proposed Mechanism of Action | Reference |
|---|---|---|---|
| A549, H460 & H1299 | Grape seed proanthocyanidins | Inhibit cell migration and endogenous nitric oxide Inhibit activation of ERK1/2 Induce apoptosis Activate caspases-9 and -3 Activate poly (ADP-ribose) polymerase | [35,36,37] |
| NCI-H209 | Quercetin glucuronides | Decrease cell viability (dose and time dependent) Arrest cell cycle at G2/M phase via caspase-3 cascade | [38] |
| A549 & H460 | Curcumin | Inhibit cell proliferation Induce fork head box protein O1 (FOXO1) expression | [39] |
| PC-9 | Curcumin | Inhibit cell growth Induce G1/S arrest via activating CDK inhibitor genes p21 and p27 | [40] |
| A549 | Polyphenol rich brown alga (Ecklonia cava) extract | Suppress migration and invasion Down-regulate MMP-2 activity Anti-metastatic effect | [41] |
| Group 1 Carcinogens | Type of Exposure |
|---|---|
| Personal Habits and Indoor Combustion | |
| Tobacco Smoking and Second Hand Smoke | E |
| Household Combustion of Coal Tar | E |
| Diesel Exhaust | E, O |
| Chemical Agents and Related Occupation | |
| benzo[a]pyrene (BaP) | O |
| Coal Gasification | O |
| Coal-tar Pitch | O |
| Coke Production | O |
| Soot (Contains BaP) | E, O |
| Aluminium Production | O |
| Bis(chloromethyl)ether and Chloromethyl Methyl Ether | O |
| Sulfur Mustard | O |
| Iron and steel founding | O |
| Painting | O |
| Rubber Manufacturing | O |
| Radiation | |
| X-radiation and γ-radiation | O |
| Internalized α-particle Emitting Radionuclides Radon (Rn)—222Rn Produced from Uranium (238U) and 220Rn Produced from Thorium Plutonium-239 | E, O |
| Metal, Fiber and Dust | |
| Arsenic and Inorganic Arsenic Compounds | E, O |
| Beryllium and its Compounds | E, O |
| Cadmium and Cadmium Compounds | E, O |
| Chromium(VI) Compounds | E, O |
| Nickel compounds | E, O |
| Asbestos | E, O |
| Crystalline silica in the form of quartz or cristobalite | E, O |
| Pharmaceuticals | |
| Mechlorethamine, Oncovin, Procarbazine, and Prednisone (MOPP) combination therapy | O |
| Animal Model | Carcinogen | Compound or Extract | Observation | Reference |
|---|---|---|---|---|
| Swiss ICR Mice | Cigarette smoke (CS) | Black chokeberry and strawberry aqueous extracts | Reduce cytogenetic damage, liver degeneration, pulmonary emphysema and lung adenomas Inhibit CS-related body weight loss | [89] |
| Mice | BaP | Hesperidin | Attenuate mast cell density Down regulate expressions of COX-2, MMP-2 and MMP-9 Exert anti-carcinogenic activity against lung cancer | [90] |
| Swiss Albino Mice | BaP | Baicalein | Increase enzyme antioxidants and non-enzyme antioxidants Decrease the activity of phase I enzymes Increase the activity of phase II detoxification enzymes Preserve pulmonary microvasculature and normal growth pattern | [91] |
| Swiss Albino mice | BaP | Mangiferin | Prevent decrement of electron transport chain complexes and TCA cycle key enzymes in lung cancer bearing mice | [92] |
| ICR Mice | Tobacco smoke | Apple polyphenol | Reduced inflammation Reverse oxidative stress in lung tissues Regulate the MMP-9 in cells | [93] |
| Swiss Albino Mice | BaP | Naringenin | Activate the enzymatic antioxidants (SOD, CAT, GPx, GST) Suppress unregulated expression of CYP1A1, PCNA and NF-κB Reduce pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) Reduce proliferative lesions in lung | [94] |
| Swiss Albino Mice | BaP | Fisetin | Restore lipid peroxidase, enzymatic and non-enzymatic antioxidants levels Reduce the lung lesions Reduce PCNA | [95] |
| A/J Mice | NNK | EGCG | Attenuate the induction of DNMT1 Reduce phospho-histone H2AX (γ-H2AX) and phospho-AKT (p-AKT) | [96] |
| Sprague-Dawley Rats | NNK | Cape gooseberry extract | Reduce pulmonary hyperplasia Improve the DNA content Reduce expression of cell proliferation marker Ki-67 Enhance expression of tumor suppressor gene p53 | [97] |
| Mongolian Gerbils | BaP | Quercetin | Suppress the expression of TNF-α, IL-1β, phospho-c-Jun and phospho-JNK | [98] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amararathna, M.; Johnston, M.R.; Rupasinghe, H.P.V. Plant Polyphenols as Chemopreventive Agents for Lung Cancer. Int. J. Mol. Sci. 2016, 17, 1352. https://doi.org/10.3390/ijms17081352
Amararathna M, Johnston MR, Rupasinghe HPV. Plant Polyphenols as Chemopreventive Agents for Lung Cancer. International Journal of Molecular Sciences. 2016; 17(8):1352. https://doi.org/10.3390/ijms17081352
Chicago/Turabian StyleAmararathna, Madumani, Michael R. Johnston, and H. P. Vasantha Rupasinghe. 2016. "Plant Polyphenols as Chemopreventive Agents for Lung Cancer" International Journal of Molecular Sciences 17, no. 8: 1352. https://doi.org/10.3390/ijms17081352
APA StyleAmararathna, M., Johnston, M. R., & Rupasinghe, H. P. V. (2016). Plant Polyphenols as Chemopreventive Agents for Lung Cancer. International Journal of Molecular Sciences, 17(8), 1352. https://doi.org/10.3390/ijms17081352