Perillyl Alcohol and Its Drug-Conjugated Derivatives as Potential Novel Methods of Treating Brain Metastases
Abstract
:1. Introduction
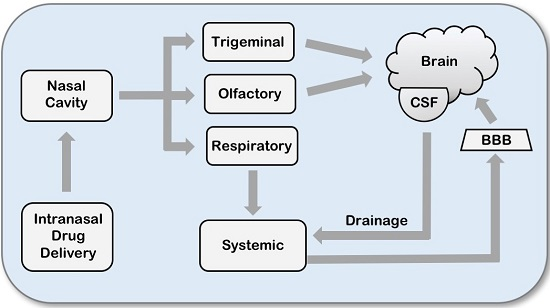
2. Intranasal Drug Delivery
2.1. Olfactory Pathway
2.2. Trigeminal Nerve Pathway
2.3. Intranasal Delivery of Cancer Drugs
2.4. Intranasal Delivery of Perillyl Alcohol
3. Perillyl Alcohol-Drug Conjugation for Improved Blood-Brain Barrier (BBB) Penetration
3.1. In Silico Prediction of BBB Penetration
3.2. Preclinical Validation of In Silico Prediction
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Walker, A.E.; Robins, M.; Weinfeld, F.D. Epidemiology of brain tumors: The national survey of intracranial neoplasms. Neurology 1985, 35, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Percy, A.K.; Elveback, L.R.; Okazaki, H.; Kurland, L.T. Neoplasms of the central nervous system. Epidemiologic considerations. Neurology 1972, 22, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovic, I.T.; Posner, J.B. Brain metastases: Epidemiology and pathophysiology. J. Neurooncol. 2005, 75, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Weathers, T.; Haney, L.G.; Timmerman, R.; Dickler, M.; Shen, J.; Sledge, G.W., Jr. Occult central nervous system involvement in patients with metastatic breast cancer: Prevalence, predictive factors and impact on overall survival. Ann. Oncol. 2003, 14, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Posner, J.B.; Chernik, N.L. Intracranial metastases from systemic cancer. Adv. Neurol. 1978, 19, 579–592. [Google Scholar] [PubMed]
- Tsukada, Y.; Fouad, A.; Pickren, J.W.; Lane, W.W. Central nervous system metastasis from breast carcinoma autopsy study. Cancer 1983, 52, 2349–2354. [Google Scholar] [CrossRef]
- Davis, F.G.; Dolecek, T.A.; McCarthy, B.J.; Villano, J.L. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro-Oncology 2012, 14, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S.; Camphausen, K.A.; Smith, Q.R. Brain metastases as preventive and therapeutic targets. Nat. Rev. Cancer 2011, 11, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.; Neman, J. Role of Blood-Brain Barrier, Choroid Plexus, and Cerebral Spinal Fluid in Extravasation and Colonization of Brain Metastases. In The Choroid Plexus and Cerebrospinal Fluid—Emerging Roles in CNS Development, Maintenance, and Disease Progression; Neman, J., Chen, T.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–102. [Google Scholar]
- Demopoulos, A. Leptomeningeal metastases. Curr. Neurol. Neurosci. Rep. 2004, 4, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.J.; Rutten, J.; Huveneers, H.A.; Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002, 94, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Drappatz, J.; Batchelor, T.T. Leptomeningeal neoplasms. Curr. Treat. Options Neurol. 2007, 9, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Taillibert, S.; Chamberlain, M.C. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg. Neurol. Int. 2013, 4 (Suppl. S4), S265–S288. [Google Scholar] [PubMed]
- Lee, D.W.; Lee, K.H.; Kim, J.W.; Keam, B. Molecular Targeted Therapies for the Treatment of Leptomeningeal Carcinomatosis: Current Evidence and Future Directions. Int. J. Mol. Sci. 2016, 17, 1074. [Google Scholar] [CrossRef] [PubMed]
- Mack, F.; Baumert, B.G.; Schafer, N.; Hattingen, E.; Scheffler, B.; Herrlinger, U.; Glas, M. Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat. Rev. 2016, 43, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, E.S.; Djalilian, H.R.; Cho, K.H.; Hall, W.A. Brain metastases: Histology, multiplicity, surgery, and survival. Cancer 1996, 78, 1781–1788. [Google Scholar] [CrossRef]
- Quigley, M.R.; Fukui, O.; Chew, B.; Bhatia, S.; Karlovits, S. The shifting landscape of metastatic breast cancer to the CNS. Neurosurg. Rev. 2013, 36, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The role of the organ microenvironment in brain metastasis. Semin. Cancer Biol. 2011, 21, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Mehta, M.P. Targeted therapy for brain metastases: Improving the therapeutic ratio. Clin. Cancer Res. 2007, 13, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.G.; Pricola, K.; Sarmiento, J.M.; Garg, S.K.; Bryant, A.; Black, K.L. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst. Rev. 2012, 6, CD006121. [Google Scholar]
- Bartsch, R.; Berghoff, A.S.; Preusser, M. Optimal management of brain metastases from breast cancer. Issues and considerations. CNS Drugs 2013, 27, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Grosu, A.L.; Gaspar, L.E. Stereotactic radiosurgery (SRS) for brain metastases: A systematic review. Radiat. Oncol. 2014, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-Year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, L.; Qian, J.Q.; Qiu, T.Z.; Shu, Y.Q.; Liu, P. Temozolomide for treatment of brain metastases: A review of 21 clinical trials. World J. Clin. Oncol. 2014, 5, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.I.; Lebas, N.; Gerber, S.; Levy, C.; Le Scodan, R.; Bourgier, C.; Pierga, J.Y.; Gobillion, A.; Savignoni, A.; Kirova, Y.M. Phase II randomized study of whole-brain radiation therapy with or without concurrent temozolomide for brain metastases from breast cancer. Ann. Oncol. 2015, 26, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Tatar, Z.; Thivat, E.; Planchat, E.; Gimbergues, P.; Gadea, E.; Abrial, C.; Durando, X. Temozolomide and unusual indications: Review of literature. Cancer Treat. Rev. 2013, 39, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Duchnowska, R.; Woditschka, S.; Hua, E.; Qian, Y.; Biernat, W.; Sosinska-Mielcarek, K.; Gril, B.; Stark, A.M.; Hewitt, S.M.; et al. Profound prevention of experimental brain metastases of breast cancer by temozolomide in an MGMT-dependent manner. Clin. Cancer Res. 2014, 20, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Deeken, J.F.; Loscher, W. The blood-brain barrier and cancer: Transporters, treatment, and Trojan horses. Clin. Cancer Res. 2007, 13, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Tsuta, K.; Ono, M.; Shimizu, C.; Hirakawa, A.; Hasegawa, T.; Hatanaka, Y.; Narita, Y.; Shibui, S.; Fujiwara, Y. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer 2010, 116, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Boogerd, W.; Dalesio, O.; Bais, E.M.; van der Sande, J.J. Response of brain metastases from breast cancer to systemic chemotherapy. Cancer 1992, 69, 972–980. [Google Scholar] [CrossRef]
- Boogerd, W.; Groenveld, F.; Linn, S.; Baars, J.W.; Brandsma, D.; van Tinteren, H. Chemotherapy as primary treatment for brain metastases from breast cancer: Analysis of 115 one-year survivors. J. Cancer Res. Clin. Oncol. 2012, 138, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, V.; Cocconi, G.; Michiara, M.; di Costanzo, F.; Fosser, V.; Tonato, M.; Carlini, P.; Boni, C.; di Sarra, S. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: A prospective study. Cancer 1999, 85, 1599–1605. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef] [PubMed]
- Arslan, C.; Dizdar, O.; Altundag, K. Systemic treatment in breast-cancer patients with brain metastasis. Expert Opin. Pharmacother. 2010, 11, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Leone, J.P.; Leone, B.A. Breast cancer brain metastases: The last frontier. Exp. Hematol. Oncol. 2015, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Lin, N.U. Updates on the management of breast cancer brain metastases. Oncology 2014, 28, 572–578. [Google Scholar] [PubMed]
- Dickson, P.I. Novel treatments and future perspectives: Outcomes of intrathecal drug delivery. Int. J. Clin. Pharmacol. Ther. 2009, 47 (Suppl. S1), S124–S127. [Google Scholar] [PubMed]
- Aiello-Laws, L.; Rutledge, D.N. Management of adult patients receiving intraventricular chemotherapy for the treatment of leptomeningeal metastasis. Clin. J. Oncol. Nurs. 2008, 12, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K. Drug delivery systems, CNS protection, and the blood brain barrier. BioMed Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Gomes, B.; Coelho, M.A.; do Carmo Pereira, M.; Rocha, S. Targeting nanoparticles across the blood-brain barrier with monoclonal antibodies. Nanomedicine 2014, 9, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M.; Martin, D.R.; Byrne, M.E. Recent advances in delivery through the blood-brain barrier. Curr. Top. Med. Chem. 2014, 14, 1148–1160. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nasal drug delivery—Recent developments and future prospects. J. Control. Release 2012, 161, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Gizurarson, S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr. Drug Deliv. 2012, 9, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, T.R.; Bernstone, T. Intranasal drug delivery: An alternative to intravenous administration in selected emergency cases. J. Emerg. Nurs. 2004, 30, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Bitter, C.; Suter-Zimmermann, K.; Surber, C. Nasal drug delivery in humans. Curr. Probl. Dermatol. 2011, 40, 20–35. [Google Scholar] [PubMed]
- Ugwoke, M.I.; Agu, R.U.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef] [PubMed]
- Merkus, F.W.; van den Berg, M.P. Can nasal drug delivery bypass the blood-brain barrier? Questioning the direct transport theory. Drugs R D 2007, 8, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, L.; Abou-Kaoud, M.; Stepensky, D. Quantitative analysis of drug delivery to the brain via nasal route. J. Control. Release 2014, 189, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., II. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G.; Messina, J.C.; Mahmoud, R.A. The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview. Ther. Deliv. 2014, 5, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Badhan, R.K.; Kaur, M.; Lungare, S.; Obuobi, S. Improving brain drug targeting through exploitation of the nose-to-brain route: A physiological and pharmacokinetic perspective. Curr. Drug Deliv. 2014, 11, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Van Woensel, M.; Wauthoz, N.; Rosiere, R.; Amighi, K.; Mathieu, V.; Lefranc, F.; van Gool, S.W.; de Vleeschouwer, S. Formulations for Intranasal Delivery of Pharmacological Agents to Combat Brain Disease: A New Opportunity to Tackle GBM? Cancers 2013, 5, 1020–1048. [Google Scholar] [CrossRef] [PubMed]
- Jansson, B.; Bjork, E. Visualization of in vivo olfactory uptake and transfer using fluorescein dextran. J. Drug Target 2002, 10, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Leopold, D.A. The relationship between nasal anatomy and human olfaction. Laryngoscope 1988, 98, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Jafek, B.W. Ultrastructure of human nasal mucosa. Laryngoscope 1983, 93, 1576–1599. [Google Scholar] [CrossRef] [PubMed]
- Lledo, P.M.; Gheusi, G.; Vincent, J.D. Information processing in the mammalian olfactory system. Phys. Rev. 2005, 85, 281–317. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H., II. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.J.; Hanson, L.R.; Frey, W.H. Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol. Pharm. 2010, 7, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Frey, W.H., II. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. S3), S5. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Martinez, J.A.; Hanson, L.R.; Frey, W.H., II; Toth, C.C. Intranasal treatment of neurodegenerative diseases and stroke. Front. Biosci. 2012, 4, 74–89. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Liu, X. Intranasal delivery: Circumventing the iron curtain to treat neurological disorders. Expert Opin. Drug Deliv. 2015, 12, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Kalviainen, R. Intranasal therapies for acute seizures. Epilepsy Behav. 2015, 49, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Ali, A.; Md, S.; Baboota, S.; Sahni, J.K.; Ali, J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2014, 21, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef]
- Salameh, T.S.; Bullock, K.M.; Hujoel, I.A.; Niehoff, M.L.; Wolden-Hanson, T.; Kim, J.; Morley, J.E.; Farr, S.A.; Banks, W.A. Central Nervous System Delivery of Intranasal Insulin: Mechanisms of Uptake and Effects on Cognition. J. Alzheimers Dis. 2015, 47, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.E.; Waszczak, B.L. Intranasal gene delivery for treating Parkinson’s disease: Overcoming the blood-brain barrier. Expert Opin. Drug Deliv. 2015, 12, 1923–1941. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, C.; Kundu, P.; Sahoo, S.K. Brain Targeting of siRNA via Intranasal Pathway. Curr. Pharm. Des. 2015, 21, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.A.; Hanson, L.R.; Aronovich, E.L.; Nan, Z.; Low, W.C.; Frey, W.H., II; McIvor, R.S. Lysosomal enzyme can bypass the blood-brain barrier and reach the CNS following intranasal administration. Mol. Genet. Metab. 2012, 106, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhu, J.; Xu, G.; Liu, X. Intranasal delivery of stem cells to the brain. Expert Opin. Drug Deliv. 2011, 8, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Feng, L.; Zhang, G.X.; Ma, C.G. Intranasal delivery of stem cells as therapy for central nervous system disease. Exp. Mol. Pathol. 2015, 98, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Fawcett, J.R.; Rahman, Y.E.; Ala, T.A.; Frey, I.W. Delivery of Nerve Growth Factor to the Brain via the Olfactory Pathway. J. Alzheimers Dis. 1998, 1, 35–44. [Google Scholar] [PubMed]
- Fliedner, S.; Schulz, C.; Lehnert, H. Brain uptake of intranasally applied radioiodinated leptin in Wistar rats. Endocrinology 2006, 147, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jiang, X.; Lu, W. Profiles of methotrexate in blood and CSF following intranasal and intravenous administration to rats. Int. J. Pharm. 2003, 263, 1–7. [Google Scholar] [CrossRef]
- Stevens, J.; Ploeger, B.A.; van der Graaf, P.H.; Danhof, M.; de Lange, E.C. Systemic and direct nose-to-brain transport pharmacokinetic model for remoxipride after intravenous and intranasal administration. Drug Metab. Dispos. 2011, 39, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Serwer, L.P.; James, C.D. Challenges in drug delivery to tumors of the central nervous system: An overview of pharmacological and surgical considerations. Adv. Drug Deliv. Rev. 2012, 64, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Bansal, A.; Hofman, F.; Chen, T.C.; Zada, G. A systematic review of inhaled intranasal therapy for central nervous system neoplasms: An emerging therapeutic option. J. Neurooncol. 2014, 116, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, Y.; Yun, L. Study on brain targeting of raltitrexed following intranasal administration in rats. Cancer Chemother. Pharmacol. 2006, 57, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Shingaki, T.; Hidalgo, I.J.; Furubayashi, T.; Katsumi, H.; Sakane, T.; Yamamoto, A.; Yamashita, S. The transnasal delivery of 5-fluorouracil to the rat brain is enhanced by acetazolamide (the inhibitor of the secretion of cerebrospinal fluid). Int. J. Pharm. 2009, 377, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sakane, T.; Yamashita, S.; Yata, N.; Sezaki, H. Transnasal delivery of 5-fluorouracil to the brain in the rat. J. Drug Target 1999, 7, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Shingaki, T.; Inoue, D.; Furubayashi, T.; Sakane, T.; Katsumi, H.; Yamamoto, A.; Yamashita, S. Transnasal delivery of methotrexate to brain tumors in rats: A new strategy for brain tumor chemotherapy. Mol. Pharm. 2010, 7, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Blakeley, J.O.; Olson, J.; Grossman, S.A.; He, X.; Weingart, J.; Supko, J.G.; New Approaches to Brain Tumor Therapy (NABTT) Consortium. Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: A microdialysis study. J. Neurooncol. 2009, 91, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, R.; Ozawa, T.; Gryaznov, S.M.; Bollen, A.W.; Lamborn, K.R.; Frey, W.H., II; Deen, D.F. New therapeutic approach for brain tumors: Intranasal delivery of telomerase inhibitor GRN163. Neuro-Oncology 2008, 10, 112–120. [Google Scholar] [CrossRef]
- Taki, H.; Kanazawa, T.; Akiyama, F.; Takashima, Y.; Okada, H. Intranasal delivery of camptothecin-loaded Tat-modified nanomicells for treatment of intracranial brain tumors. Pharmaceuticals 2012, 5, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.L.; Elson, C.E. Isoprenoids, Health and Disease. In Nutraceuticals and Functional Foods; Wildman, R.E.C., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 31–54. [Google Scholar]
- Haag, J.D.; Gould, M.N. Mammary carcinoma regression induced by perillyl alcohol, a hydroxylated analog of limonene. Cancer Chemother. Pharmacol. 1994, 34, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.J.; Chari, R.S.; Boyer, I.J.; Gould, M.N.; Jirtle, R.L. Induction of apoptosis in liver tumors by the monoterpene perillyl alcohol. Cancer Res. 1995, 55, 979–983. [Google Scholar] [PubMed]
- Ong, T.P.; Cardozo, M.T.; de Conti, A.; Moreno, F.S. Chemoprevention of hepatocarcinogenesis with dietary isoprenic derivatives: Cellular and molecular aspects. Curr. Cancer Drug Targets 2012, 12, 1173–1190. [Google Scholar] [PubMed]
- Stark, M.J.; Burke, Y.D.; McKinzie, J.H.; Ayoubi, A.S.; Crowell, P.L. Chemotherapy of pancreatic cancer with the monoterpene perillyl alcohol. Cancer Lett. 1995, 96, 15–21. [Google Scholar] [CrossRef]
- Yuri, T.; Danbara, N.; Tsujita-Kyutoku, M.; Kiyozuka, Y.; Senzaki, H.; Shikata, N.; Kanzaki, H.; Tsubura, A. Perillyl alcohol inhibits human breast cancer cell growth in vitro and in vivo. Breast Cancer Res. Treat. 2004, 84, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Teruszkin Balassiano, I.; Alves de Paulo, S.; Henriques Silva, N.; Curie Cabral, M.; Gibaldi, D.; Bozza, M.; Orlando da Fonseca, C.; Da Gloria da Costa Carvalho, M. Effects of perillyl alcohol in glial C6 cell line in vitro and anti-metastatic activity in chorioallantoic membrane model. Int. J. Mol. Med. 2002, 10, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Azzoli, C.G.; Miller, V.A.; Ng, K.K.; Krug, L.M.; Spriggs, D.R.; Tong, W.P.; Riedel, E.R.; Kris, M.G. A phase I trial of perillyl alcohol in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2003, 51, 493–498. [Google Scholar] [PubMed]
- Bailey, H.H.; Attia, S.; Love, R.R.; Fass, T.; Chappell, R.; Tutsch, K.; Harris, L.; Jumonville, A.; Hansen, R.; Shapiro, G.R.; et al. Phase II trial of daily oral perillyl alcohol (NSC 641066) in treatment-refractory metastatic breast cancer. Cancer Chemother. Pharmacol. 2008, 62, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.H.; Wilding, G.; Tutsch, K.D.; Arzoomanian, R.Z.; Alberti, D.; Feierabend, C.; Simon, K.; Marnocha, R.; Holstein, S.A.; Stewart, J.; et al. A phase I trial of perillyl alcohol administered four times daily for 14 days out of 28 days. Cancer Chemother. Pharmacol. 2004, 54, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Oettel, K.; Bailey, H.; Ummersen, L.V.; Tutsch, K.; Staab, M.J.; Horvath, D.; Alberti, D.; Arzoomanian, R.; Rezazadeh, H.; et al. Phase II trial of perillyl alcohol (NSC 641066) administered daily in patients with metastatic androgen independent prostate cancer. Investig. New Drugs 2003, 21, 367–372. [Google Scholar] [CrossRef]
- Morgan-Meadows, S.; Dubey, S.; Gould, M.; Tutsch, K.; Marnocha, R.; Arzoomanin, R.; Alberti, D.; Binger, K.; Feierabend, C.; Volkman, J.; et al. Phase I trial of perillyl alcohol administered four times daily continuously. Cancer Chemother. Pharmacol. 2003, 52, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Murren, J.R.; Pizzorno, G.; DiStasio, S.A.; McKeon, A.; Peccerillo, K.; Gollerkari, A.; McMurray, W.; Burtness, B.A.; Rutherford, T.; Li, X.; et al. Phase I study of perillyl alcohol in patients with refractory malignancies. Cancer Biol. Ther. 2002, 1, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Ripple, G.H.; Gould, M.N.; Arzoomanian, R.Z.; Alberti, D.; Feierabend, C.; Simon, K.; Binger, K.; Tutsch, K.D.; Pomplun, M.; Wahamaki, A.; et al. Phase I clinical and pharmacokinetic study of perillyl alcohol administered four times a day. Clin. Cancer Res. 2000, 6, 390–396. [Google Scholar] [PubMed]
- Chen, T.C.; Fonseca, C.O.; Schönthal, A.H. Preclinical development and clinical use of perillyl alcohol for chemoprevention and cancer therapy. Am. J. Cancer Res. 2015, 5, 1580–1593. [Google Scholar] [PubMed]
- Cho, H.Y.; Wang, W.; Jhaveri, N.; Torres, S.; Tseng, J.; Leong, M.N.; Lee, D.J.; Goldkorn, A.; Xu, T.; Petasis, N.A.; et al. Perillyl alcohol for the treatment of temozolomide-resistant gliomas. Mol. Cancer Ther. 2012, 11, 2462–2472. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, C.O.; Schwartsmann, G.; Fischer, J.; Nagel, J.; Futuro, D.; Quirico-Santos, T.; Gattass, C.R. Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg. Neurol. 2008, 70, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, C.O.; Teixeira, R.M.; Silva, J.C.; de Saldanha da Gama Fischer, J.; Meirelles, O.C.; Landeiro, J.A.; Quirico-Santos, T. Long-term outcome in patients with recurrent malignant glioma treated with perillyl alcohol inhalation. Anticancer Res. 2013, 33, 5625–5631. [Google Scholar] [PubMed]
- Da Fonseca, C.O.; Quirico-Santos, T. Perillyl Alcohol: A Pharmacotherapeutic Report. In Bioactive Essential Oils and Cancer; de Sousa, D.P., Ed.; Springer International Publishing Switzerland: Heidelberg, Germany; New York, NY, USA; London, UK, 2015; pp. 267–288. [Google Scholar]
- Da Fonseca, C.O.; Simao, M.; Lins, I.R.; Caetano, R.O.; Futuro, D.; Quirico-Santos, T. Efficacy of monoterpene perillyl alcohol upon survival rate of patients with recurrent glioblastoma. J. Cancer Res. Clin. Oncol. 2011, 137, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef] [PubMed]
- Vastag, M.; Keseru, G.M. Current in vitro and in silico models of blood-brain barrier penetration: A practical view. Curr. Opin. Drug Discov. Dev. 2009, 12, 115–124. [Google Scholar]
- Geldenhuys, W.J.; Mohammad, A.S.; Adkins, C.E.; Lockman, P.R. Molecular determinants of blood-brain barrier permeation. Ther. Deliv. 2015, 6, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.T.; Clark, D.E. In silico predictions of blood-brain barrier penetration: Considerations to “keep in mind”. J. Pharmacol. Exp. Ther. 2005, 315, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, A.R.; Hamidi, M. Brain drug targeting: A computational approach for overcoming blood-brain barrier. Drug Discov. Today 2009, 14, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Lanevskij, K.; Japertas, P.; Didziapetris, R.; Petrauskas, A. Ionization-specific prediction of blood-brain permeability. J. Pharm. Sci. 2009, 98, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Bafaloukos, D.; Kosmidis, P.; Samantas, E.; Bamias, A.; Papakostas, P.; Karabelis, A.; Bacoyiannis, C.; Skarlos, D.V. Phase II study of temozolomide in heavily pretreated cancer patients with brain metastases. Ann. Oncol. 2001, 12, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Abrey, L.E.; Olson, J.D.; Raizer, J.J.; Mack, M.; Rodavitch, A.; Boutros, D.Y.; Malkin, M.G. A phase II trial of temozolomide for patients with recurrent or progressive brain metastases. J. Neurooncol. 2001, 53, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, M.E.; Crump, M.; Charpentier, D.; Yelle, L.; Bordeleau, L.; Matthews, S.; Eisenhauer, E. Temozolomide in metastatic breast cancer (MBC): A phase II trial of the National Cancer Institute of Canada—Clinical Trials Group (NCIC-CTG). Ann. Oncol. 2006, 17, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Addeo, R.; de Rosa, C.; Faiola, V.; Leo, L.; Cennamo, G.; Montella, L.; Guarrasi, R.; Vincenzi, B.; Caraglia, M.; del Prete, S. Phase 2 trial of temozolomide using protracted low-dose and whole-brain radiotherapy for nonsmall cell lung cancer and breast cancer patients with brain metastases. Cancer 2008, 113, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Crino, L.; Danova, M.; Del Prete, S.; Cascinu, S.; Salvagni, S.; Schiavetto, I.; Vitali, M.; Bajetta, E. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: A multicenter phase II study. Ann. Oncol. 2010, 21, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Addeo, R.; Sperlongano, P.; Montella, L.; Vincenzi, B.; Carraturo, M.; Iodice, P.; Russo, P.; Parlato, C.; Salzano, A.; Cennamo, G.; et al. Protracted low dose of oral vinorelbine and temozolomide with whole-brain radiotherapy in the treatment for breast cancer patients with brain metastases. Cancer Chemother. Pharmacol. 2012, 70, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Wang, W.; Jhaveri, N.; Lee, D.J.; Sharma, N.; Dubeau, L.; Schönthal, A.H.; Hofman, F.M.; Chen, T.C. NEO212, temozolomide conjugated to perillyl alcohol, is a novel drug for effective treatment of a broad range of temozolomide-resistant gliomas. Mol. Cancer Ther. 2014, 13, 2004–2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Cho, H.Y.; Wang, W.; Barath, M.; Sharma, N.; Hofman, F.M.; Schönthal, A.H. A novel temozolomide-perillyl alcohol conjugate exhibits superior activity against breast cancer cells in vitro and intracranial triple-negative tumor growth in vivo. Mol. Cancer Ther. 2014, 13, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Swenson, S.; Hofman, F.M.; Schönthal, A.H.; Chen, T.C. Brain/plasma ratios of NEO212 in vivo. Unpublished work. 2016. [Google Scholar]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.C.; Da Fonseca, C.O.; Schönthal, A.H. Perillyl Alcohol and Its Drug-Conjugated Derivatives as Potential Novel Methods of Treating Brain Metastases. Int. J. Mol. Sci. 2016, 17, 1463. https://doi.org/10.3390/ijms17091463
Chen TC, Da Fonseca CO, Schönthal AH. Perillyl Alcohol and Its Drug-Conjugated Derivatives as Potential Novel Methods of Treating Brain Metastases. International Journal of Molecular Sciences. 2016; 17(9):1463. https://doi.org/10.3390/ijms17091463
Chicago/Turabian StyleChen, Thomas C., Clovis O. Da Fonseca, and Axel H. Schönthal. 2016. "Perillyl Alcohol and Its Drug-Conjugated Derivatives as Potential Novel Methods of Treating Brain Metastases" International Journal of Molecular Sciences 17, no. 9: 1463. https://doi.org/10.3390/ijms17091463
APA StyleChen, T. C., Da Fonseca, C. O., & Schönthal, A. H. (2016). Perillyl Alcohol and Its Drug-Conjugated Derivatives as Potential Novel Methods of Treating Brain Metastases. International Journal of Molecular Sciences, 17(9), 1463. https://doi.org/10.3390/ijms17091463