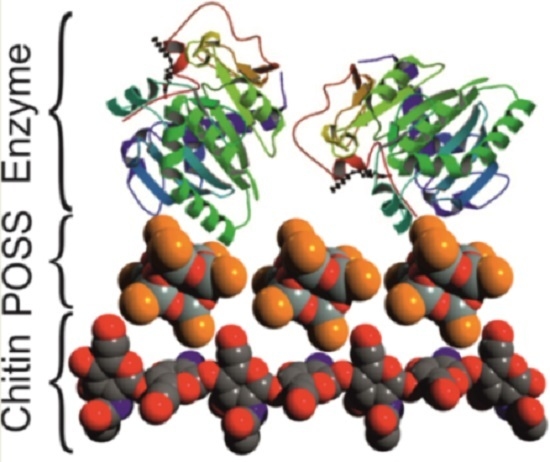
Candida antarctica Lipase B Immobilized onto Chitin Conjugated with POSS® Compounds: Useful Tool for Rapeseed Oil Conversion
Abstract
:1. Introduction
2. Results
2.1. Chitin Surface Functionalization
2.1.1. Surface Analysis
2.1.2. Raman Spectroscopy Results
2.1.3. Thermogravimetric Analysis of the Chitin-POSS Materials
2.2. Characterization of Products Following Lipase Immobilization
2.2.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of the Products Following Immobilization
2.2.2. Retention of Hydrolytic Activity of Products after Immobilization
2.2.3. Evaluation of the Stability of the Products after Immobilization
2.2.4. Rapeseed Oil Transesterification with Immobilized CALB
3. Discussion
3.1. Chitin Surface Functionalization
3.1.1. XPS Analysis
3.1.2. Raman Spectroscopy
3.1.3. Thermal Stability Analysis
3.2. Characterization of Products Following Lipase Immobilization
3.2.1. FTIR Analysis
3.2.2. Retention of Hydrolytic Activity of Products after Immobilization
3.2.3. Effect of the Various Reaction Conditions on the Stability of the Products after Immobilization
3.2.4. Rapeseed Oil Transesterification with Immobilized CALB
3.2.5. Suggested Mechanism of the Chitin Modification and Enzyme Immobilization
4. Materials and Methods
4.1. Reagents
4.2. Functionalization of the Chitin Surface
4.3. Immobilization of Lipase onto the Modified Chitin Matrix
4.4. Experimental Techniques
4.5. Evaluation of Hydrolytic Activity of Lipase after Immobilization
4.5.1. Products after Immobilization Stability
4.5.2. Storage Stability and Reusability
4.6. Methanolysis Reaction of the Rapeseed Oil
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brunner, E.; Richthammer, P.; Ehrlich, H.; Paasch, S.; Simon, P.; Ueberlein, S.; van Pée, K.H. Chitin-based organic networks: An integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew. Chem. Int. Ed. 2009, 48, 9724–9727. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, Y.; Yu, L.; Zhang, C.; Xu, X.; Xue, Y.; Li, Z.; Xue, C. Crystalline structure and thermal property characterization of chitin from Antarctic krill (Euphausia superba). Carbohydr. Polym. 2013, 92, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T. Description of a new surface morphology for chitin extracted from wings of cockroach (Periplaneta americana). Int. J. Biol. Macromol. 2015, 75, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Baran, T.; Erdogan, S.; Mentes, A.; Ozusaglam, M.A.; Cakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C 2014, 45, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Maldonado, M.; Spindler, K.D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Kaluzhnaya, O.V.; Brunner, E.; Tsurkan, M.V.; Ereskovsky, A.; Ilan, M.; Tabachnick, K.R.; Bazhenov, V.V.; Paasch, S.; Kammer, M.; et al. Identification and first insights into the structure and biosynthesis of chitin from the freshwater sponge Spongilla lacustris. J. Struct. Biol. 2013, 183, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Zall, R.R. Absorption of metals by natural polymers generated from seafood processing wastes. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 168–172. [Google Scholar] [CrossRef]
- Kurita, K. Controlled functionalization of the poly-saccharide chitin. Prog. Polym. Sci. 2001, 26, 1921–1971. [Google Scholar] [CrossRef]
- Ehrlich, H.; Rigby, J.K.; Botting, J.P.; Tsurkan, M.V.; Werner, C.; Schwille, P.; Petrasek, Z.; Pisera, A.; Simon, P.; Sivkov, V.N.; et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Wysokowski, M.; Majchrzak, I.; Szatkowski, T.; Nowacka, M.; Siwińska-Stefańska, K.; Szwarc-Rzepka, K.; Bartczak, P.; Ehrlich, H.; Jesionowski, T. Preparation and characterization of multifunctional chitin/lignin materials. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Wysokowski, M.; Klapiszewski, L.; Moszynski, D.; Bartczak, P.; Szatkowski, T.; Majchrzak, I.; Siwinska-Stefanska, K.; Bazhenov, V.V.; Jesionowski, T. Modification of chitin with kraft lignin and development of new biosorbents for removal of cadmium(II) and nickel(II) ions. Mar. Drugs 2014, 12, 2245–2268. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Sudheesh Kumar, P.T.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 2013, 113, 5458–5479. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Simon, P.; Motylenko, M.; Wysokowski, M.; Bazhenov, V.V.; Galli, R.; Stelling, A.L.; Stawski, D.; Ilan, M.; Stöcker, H.; et al. Extreme biomimetics: Formation of zirconium dioxide nanophase using chitinous scaffolds under hydrothermal conditions. J. Mater. Chem. B 2013, 1, 5092–5099. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Walter, J.; Lota, G.; Wojciechowski, J.; Stöcker, H.; Galli, R.; Stelling, A.L.; Himcinschi, C.; Niederschlag, E.; et al. Synthesis of nanostructured chitin-hematite composites under extreme biomimetic conditions. RSC Adv. 2014, 4, 61743–61752. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Lewicki, J.; Pielichowski, K.; Jancia, M.; Hebda, E.; Albo, R.L.F.; Maxwell, R.S. Degradative and morphological characterization of POSS modified nanohybrid polyurethane elastomers. Polym. Degrad. Stabil. 2014, 104, 50–56. [Google Scholar] [CrossRef]
- Tishchenko, G.; Bleha, M. Diffusion permeability of hybrid chitosan/polyhedral oligomeric silsesquioxanes (POSS™) membranes to amino acids. J. Membr. Sci. 2005, 248, 45–51. [Google Scholar] [CrossRef]
- Wysokowski, M.; Materna, K.; Walter, J.; Petrenko, I.; Stelling, A.L.; Bazhenov, V.V.; Klapiszewski, Ł.; Szatkowski, T.; Lewandowska, O.; Stawski, D.; et al. Solvothermal synthesis of hydrophobic chitin–polyhedral oligomeric silsesquioxane (POSS) nanocomposites. Int. J. Biol. Macromol. 2015, 78, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.T.N.; Fojan, P.; Petersen, S.B. How do lipases and esterases work: The electrostatic contribution. J. Biotechnol. 2001, 85, 115–147. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Silva, N.C.A.; Mirandaa, J.S.; Bolina, I.C.A.; Silva, W.C.; Hiratac, D.B.; de Castro, H.F.; Mendes, A.A. Immobilization of porcine pancreatic lipase on poly-hydroxybutyrate particles for the production of ethyl esters from macaw palm oils and pineapple flavor. Biochem. Eng. J. 2014, 82, 139–149. [Google Scholar] [CrossRef]
- Hernandez, K.; Garcia-Galan, C.; Fernández-Lafuente, R. Simple and efficient immobilization of lipase B from Candida antarctica on porous styrene-divinylbenzene beads. Enzym. Microb. Technol. 2011, 49, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.A.; Rodrigues, D.S.; Filice, M.; Fernández-Lafuente, R.; Guisán, J.M.; Palomo, J.M. Regioselective monohydrolysis of per-O-acetylated-1-substituted-β-glucopyranosides catalyzed by immobilized lipases. Tetrahedron 2008, 64, 10721–10727. [Google Scholar] [CrossRef]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef] [PubMed]
- Dicosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Ansorge-Schumacher, M.B.; Thum, O. Immobilised lipases in the cosmetics industry. Chem. Soc. Rev. 2013, 42, 6475–6490. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, C.; Luna, D.; Posadillo, A.; Sancho, E.D.; Rodríguez, S.; Bautista, F.; Luque, R.; Marinas, J.M.; Romero, A.A. Production of a new second generation biodiesel with a low cost lipase derived from Thermomyces lanuginosus: Optimization by response surface methodology. Catal. Today 2011, 167, 107–112. [Google Scholar] [CrossRef]
- Calero, J.; Cumplido, G.; Luna, D.; Sancho, E.D.; Luna, C.; Posadillo, A.; Bautista, F.M.; Romero, A.A.; Verdugo-Escamilla, C. Production of a biofuel that keeps the glycerol as a monoglyceride by using supported KF as heterogeneous catalyst. Energies 2014, 7, 3764–3780. [Google Scholar] [CrossRef]
- Szczesna-Antczak, M.; Kubiak, A.; Antczak, T.; Bielecki, S. Enzymatic biodiesel synthesis—Key factors affecting efficiency of the process. Renew. Energy 2009, 34, 1185–1194. [Google Scholar] [CrossRef]
- Esmaeilnejad-Ahranjani, P.; Kazemeini, M.; Singh, G.; Arpanaei, A. Amine-functionalized magnetic nanocomposite particles for efficient immobilization of lipase: Effects of functional molecule size on properties of the immobilized lipase. RSC Adv. 2015, 5, 33313–33327. [Google Scholar] [CrossRef]
- Min, K.; Yoo, Y.J. Recent progress in nanobiocatalysis for enzyme immobilization and its application. Biotechnol. Bioproc. Eng. 2014, 19, 553–567. [Google Scholar] [CrossRef]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2016, 38, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how? Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzym. Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzym. Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Balkus, K.J. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011, 1, 956–968. [Google Scholar] [CrossRef]
- Balcao, V.M.; Vila, M.M.D.C. Structural and functional stabilization of protein entities: State-of-the-art. Adv. Drug Deliv. Rev. 2015, 93, 25–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszynska, D. Immobilization as a strategy for improving enzyme properties—Application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzym. Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Cui, B.K.; Wu, X.J.; Meng, G.; Liu, H.X.; Si, J. Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int. Biodeterior. Biodegrad. 2016, 110, 69–78. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, Y.; Luo, W.; Zheng, R.; Ying, X.; Wang, Z. Biocatalytic resolution of Rac-α-Ethyl-2-Oxo-pyrrolidineacetic acid methyl ester by immobilized recombinant Bacillus cereus esterase. Appl. Biochem. Biotechnol. 2016, 178, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Zdarta, J.; Jesionowski, T. Luffa cylindrica sponges as a thermally and chemically stable support for Aspergillus niger lipase. Biotechnol. Prog. 2016, 32, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, S.V.; Fakhrali, A.; Ranaei-Siadat, S.O.; Gharehaghaji, A.A.; Mazinani, S.; Dinarid, M.; Harati, J. Immobilization of acetylcholinesterase on electrospun poly (acrylic acid)/multi-walled carbon nanotube nanofibrous membranes. RSC Adv. 2015, 5, 42572–42579. [Google Scholar] [CrossRef]
- Ammann, E.M.; Gasser, C.A.; Hommes, G.; Corvini, P.F.X. Immobilization of defined laccase combinations for enhanced oxidation of phenolic contaminants. Appl. Microbiol. Biotechnol. 2014, 98, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, A.R.; Borreguero, I.; López-Belmonte, M.T.; Sinisterra, J.V. Covalent immobilization of crude and partially-purified upases onto inorganic supports: Stability and hyperactivation. Prog. Biotechnol. 1998, 15, 571–576. [Google Scholar]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Krajewska, B.; Piwowarska, Z. Free vs. chitosan-immobilized urease: Microenvironmental effects on enzyme inhibitions. Biocatal. Biotransform. 2005, 23, 225–232. [Google Scholar] [CrossRef]
- Li, J.; Cai, J.; Zhong, L.; Du, Y. Immobilization of a protease on modified chitosan beads for the depolymerization of chitosan. Carbohydr. Polym. 2012, 87, 2697–2705. [Google Scholar] [CrossRef]
- Pospikova, K.; Safarik, I. Low-cost, easy-to-prepare magnetic chitosan microparticles for enzymes immobilization. Carbohydr. Polym. 2013, 96, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.J.; Jang, Y.H.; Park, R.D.; Jung, W.J. Immobilization of chitinases from Streptomyces griseus and Paenibacillus illinoisensis on chitosan beads. Carbohydr. Polym. 2012, 88, 391–394. [Google Scholar] [CrossRef]
- Romo-Sanchez, S.; Arevalo-Villena, M.; Garcia Romero, E.; Ramirez, H.L.; Briones Perez, A. Immobilization of β-Glucosidase and its application for enhancement of aroma precursors in muscat wine. Food Bioproc. Technol. 2014, 7, 1381–1395. [Google Scholar] [CrossRef]
- Zdarta, J.; Klapiszewski, Ł.; Wysokowski, M.; Norman, M.; Kołodziejczak-Radzimska, A.; Moszyński, D.; Ehrlich, H.; Maciejewski, H.; Stelling, A.L.; Jesionowski, T. Chitin-lignin material as a novel matrix for enzyme immobilization. Mar. Drugs 2015, 13, 2424–2446. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2015, 92, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Sanjust, E. Inorganic materials as supports for covalent enzyme immobilization: Methods and mechanisms. Molecules 2015, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.B.; He, Y.S.; Li, S.L.; Wang, Y.Z. Chitin whiskers: An overview. Biomacromology 2012, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Shiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Shin, S.; Lim, C.; Hwang, D. Dopamine-mediated sclerotization of regenerated chitin in ionic liquid. Materials 2013, 6, 3826–3839. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Houde, A.; Kademi, A.; Leblanc, D. Lipases and their industrial applications: An overview. Appl. Biochem. Biotechnol. 2004, 118, 155–170. [Google Scholar] [CrossRef]
- Verma, M.L.; Barrow, C.J.; Puri, M. Nanobiotechnology as a novel paradigm for enzyme immobilisation and stabilisation with potential applications in biodiesel production. Appl. Microbiol. Biotechnol. 2013, 97, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Rezakhani, N.; Molaei rad, A.; Parivar, K.; Khayati, M.; Etemadzade, S. Immobilization of protease in biopolymers (mixture of alginate-chitosan). J. Paramed. Sci. 2014, 5, 108–113. [Google Scholar]
- Sowjanya, N.T.; Dhivya, R.; Meenakshi, K.; Vedhanayakisri, K.A. Potential applications of chitosan nanoparticles as novel support in enzyme immobilization. Res. J. Eng. Technol. 2013, 4, 288–294. [Google Scholar]
- Kilinc, A.; Teke, M.; Onal, S.; Telefoncu, A. Immobilization of pancreatic lipase on chitin and chitosan. Prep. Biochem. Biotechnol. 2006, 36, 153–163. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, L.A.; Hynes, A.; Alexander, M.R. A methodology for curve-fitting of the XPS Si 2p core level from thin siloxane coatings. Surf. Interface Anal. 2007, 39, 926–936. [Google Scholar] [CrossRef]
- Jalarvo, N.; Gourdon, O.; Ehlers, G.; Tyagi, M.; Kumar, S.K.; Dobbs, K.D.; Smalley, R.J.; Guise, W.E.; Ramirez-Cuesta, A.; Wildgruber, C.; et al. Structure and dynamics of Octamethyl-POSS nanoparticles. J. Phys. Chem. C 2014, 118, 5579–5592. [Google Scholar] [CrossRef]
- Boscher, N.D.; Choquet, P.; Duday, D.; Verdier, S. Influence of cyclic organosilicon precursors on the corrosion of aluminium coated sheet by atmospheric pressure dielectric barrier discharge. Surf. Coat. Technol. 2011, 205, 5350–5357. [Google Scholar] [CrossRef]
- Kotiaho, A.; Myllyperkio, P.; Pettersson, M. Chemically selective imaging of overlapping C–H stretching vibrations with time-resolved coherent anti-stokes Raman scattering (CARS) microscopy. J. Phys. Chem. B 2014, 118, 4363–4369. [Google Scholar] [CrossRef] [PubMed]
- Pereiera, F.S.; da Silva, A.; Job, A.E.; Gonzalez, E.R.P. Thermal studies of chitin–chitosan derivatives. J. Therm. Anal. Calorim. 2013, 114, 321–327. [Google Scholar] [CrossRef]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohydr. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
- Georgieva, V.; Zvezdova, D.; Vlaev, L. Nonisothermal kinetics of thermal degradation of chitin. J. Therm. Anal. Calorim. 2013, 111, 763–771. [Google Scholar] [CrossRef]
- Lopez, F.A.; Merce, A.L.R.; Alguacil, F.J.; Lopez-Delgado, A. A kinetic study on the thermal behaviour of chitosan. J. Therm. Anal. Calorim. 2008, 91, 633–639. [Google Scholar] [CrossRef]
- Portaccio, M.; Della Ventura, B.; Mita, D.G.; Manolova, N.; Stoilova, O.; Rashkov, I.; Lepore, M. FT-IR microscopy characterization of sol–gel layers prior and after glucose oxidase immobilization for biosensing applications. J. Sol-Gel Sci. Technol. 2011, 57, 204–211. [Google Scholar]
- Szwarc-Rzepka, K.; Marciniec, B.; Jesionowski, T. Immobilization of multifunctional silsesquioxane cage on precipitated silica supports. Adsorption 2013, 19, 483–494. [Google Scholar] [CrossRef]
- Bianchini, D.; Barsan, M.M.; Butler, I.S.; Galland, G.B.; dos Santos, J.H.Z.; Fasce, D.P.; Williams, R.J.J.; Quijada, R. Vibrational spectra of silsesquioxanes impregnated with the metallocene catalyst bis(eta(5)-cyclopentadienyl)zirconium(IV) dichloride. Spectrochim. Acta A 2007, 68, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Croce, G.; Carniato, F.; Milanesio, M.; Boccaleri, E.; Paul, G.; van Beek, W.; Marchese, L. Understanding the physico-chemical properties of polyhedral oligomeric silsesquioxanes: A variable temperature multidisciplinary study. Phys. Chem. Chem. Phys. 2009, 11, 10087–10094. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Lee, E.; Kallam, A.; Gubbins, N.; Stafslien, S.J.; Daniels, J.; Chisholm, B.J. Development of environmentally friendly, antifouling coatings based on tethered quaternary ammonium salts in a crosslinked polydimethylsiloxane matrix. J. Coat. Technol. Res. 2008, 5, 405–417. [Google Scholar] [CrossRef]
- Mikkelsen, A.; Engelsen, S.B.; Hansen, H.C.B.; Larsen, O.; Skibsted, L.H. Calcium carbonate crystallization in the α-chitin matrix of the shell of pink shrimp, Pandalus borealis, during frozen storage. J. Cryst. Growth 1997, 177, 125–134. [Google Scholar] [CrossRef]
- Svendsen, A. Lipase protein engineering. Biochim. Biophys. Acta. 2000, 1543, 223–238. [Google Scholar] [CrossRef]
- Dousseau, F.; Pezolet, M. Determination of the secondary structure content of proteins in aqueous solutions from their amide I and amide II infrared bands. Comparison between classical and partial least-squares methods. Biochemistry 1990, 29, 8771–8779. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.T.; Wong, R.K.; Caputo, T.A.; Godwin, B.; Rigas, B. Infrared spectroscopy of exfoliated human cervical cells: Evidence of extensive structural changes during carcinogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 10988–10992. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Salek, K.; Kolodziejczak-Radzimska, A.; Siwinska-Stefanska, K.; Szwarc-Rzepka, K.; Norman, M.; Klapiszewski, L.; Bartczak, P.; Kaczorek, E.; Jesionowski, T. Immobilization of Amano Lipase A onto Stöber silica surface: Process characterization and kinetic studies. Open Chem. 2015, 13, 138–148. [Google Scholar] [CrossRef]
- Ching-Ching, Y.; Yu-Ying, K.; Chien-Fu, L.; Wei-Ting, C.; Huan-Ting, W.; Tsung-Che, C.; Fan-Dan, J.; Chun-Cheng, L. Site-specific immobilization of enzymes on magnetic nanoparticles and their use in organic synthesis. Bioconj. Chem. 2012, 23, 714–724. [Google Scholar]
- Gomes, F.M.; Pereira, E.B.; de Castro, H.F. Immobilization of lipase on chitin and its use in nonconventional biocatalysis. Biomacromolecules 2004, 5, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Uzun, L.; Denizli, A. Alanine functionalized magnetic nanoparticles for reversible amyloglucosidase immobilization. Ind. Eng. Chem. Res. 2015, 54, 454–461. [Google Scholar] [CrossRef]
- Es, I.; Goncalves Vieira, J.D.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fan, F.; Wang, Y.; Feng, W.; Ji, P. Enzyme immobilization on carboxyl-functionalized graphene oxide for catalysis in organic solvent. Ind. Eng. Chem. Res. 2013, 52, 6343–6348. [Google Scholar] [CrossRef]
- Narwal, S.K.; Saun, N.K.; Gupta, R. Characterization and catalytic properties of free and silica-bound lipase: A comparative study. J. Oleo Sci. 2014, 63, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Caballero, V.; Bautista, F.M.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A.; Hidalgo, J.M.; Luque, R.; Macario, A.; Giordano, F.G. Sustainable preparation of a novel glycerol-free biofuel by using pig pancreatic lipase: Partial 1,3-regiospecific alcoholysis of sunflower oil. Proc. Biochem. 2009, 44, 334–342. [Google Scholar] [CrossRef]
- Calero, J.; Verdugo, C.; Luna, D.; Sancho, E.D.; Luna, C.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Selective ethanolysis of sunflower oil with Lipozyme RM IM, an immobilized Rhizomucor miehei lipase, to obtain a biodiesel-like biofuel, which avoids glycerol production through the monoglyceride formation. New Biotechnol. 2014, 31, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Thanh, L.T.; Okitsu, K.; Boi, L.V.; Maeda, Y. Catalytic technologies for biodiesel fuel production and utilization of glycerol: A review. Catalysts 2012, 2, 191–222. [Google Scholar] [CrossRef]
- Luna, D.; Posadillo, A.; Caballero, V.; Verdugo, C.; Bautista, F.M.; Romero, A.A.; Sancho, E.D.; Luna, C.; Calero, J. New biofuel integrating glycerol into its composition through the use of covalent immobilized pig pancreatic lipase. Int. J. Mol. Sci. 2012, 13, 10091–10112. [Google Scholar] [CrossRef] [PubMed]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; Yousefi, P.; Mohammadi, J.; Brask, J. Enzymatic production of biodiesel using lipases immobilized on silica nanoparticles as highly reusable biocatalysts: Effect of water, t-butanol and blue silica gel contents. Renew. Energy. 2016, 91, 196–206. [Google Scholar] [CrossRef]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernández-Lafuente, R.; Huguet, J.; Guisán, J.M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Armisén, P.; Sabuquillo, P.; Fernández-Lorente, G.; Guisán, J. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Palomo, J.M.; Fuentes, M.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Self-assembly of pseudomonas fluorescens lipase into bimolecular aggregates dramatically affects functional properties. Biotechnol. Bioeng. 2003, 82, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Peñas, M.M.; Fernández-Lorente, G.; Mateo, C.; Pisabarro, A.G.; Fernández-Lafuente, R.; Ramírez, L.; Guisán, J.M. Solid-phase handling of hydrophobins: Immobilized hydrophobins as a new tool to study lipases. Biomacromolecules 2003, 4, 204–210. [Google Scholar] [CrossRef] [PubMed]






| Sample Name | Surface Composition (at%) | |||
|---|---|---|---|---|
| C | O | Si | N | |
| Chitin | 62.9 | 30.8 | – | 6.2 |
| Chitin + Vinyl POSS 5.0 g | 60.7 | 21.1 | 16.6 | 1.6 |
| Chitin + Methacryl POSS 5.0 g | 51.7 | 29.0 | 18.8 | 0.4 |
| Chitin + Amino POSS 5.0 g | 57.2 | 23.5 | 17.3 | 2.0 |
| Chitin + Epoxy POSS 5.0 g | 60.5 | 23.7 | 14.9 | 0.9 |
| Sample Name | Immobilization Time (h) | Retention of Hydrolytic Activity (%) |
|---|---|---|
| Chitin + CALB 5 mg/cm3 | 1 | 71.4 ± 2.4 |
| 24 | 68.5 ± 2.3 | |
| 96 | 63.2 ± 2.6 | |
| Chitin + VPOSS + CALB 5 mg/cm3 | 1 | 74.8 ± 2.1 |
| 24 | 81.4 ± 2.2 | |
| 96 | 75.8 ± 2.1 | |
| Chitin + MPOSS + CALB 5 mg/cm3 | 1 | 78.2 ± 2.4 |
| 24 | 87.6 ± 2.3 | |
| 96 | 73.4 ± 2.0 |
| pH | Time (h) | Chitin + CALB | Chitin + VPOSS + CALB | Chitin + MPOSS + CALB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| * | ** | *** | * | ** | *** | * | ** | *** | ||
| 6 | 12 | 7.5 ± 0.1 | 2.2 ± 0.1 | 7 ± 0.1 | 9.7 ± 0.2 | 2.2 ± 0.1 | 9 ± 0.1 | 14.0 ± 0.1 | 3.2 ± 0.1 | 13 ± 0.2 |
| 24 | 11.8 ± 0.1 | 3.2 ± 0.1 | 11 ± 0.2 | 14.0 ± 0.2 | 3.2 ± 0.1 | 13 ± 0.2 | 20.4 ± 0.3 | 5.4 ± 0.2 | 19 ± 0.3 | |
| 7 | 12 | 13.9 ± 0.2 | 4.3 ± 0.1 | 13 ± 0.1 | 10.7 ± 0.2 | 3.2 ± 0.1 | 10 ± 0.2 | 16.2 ± 0.2 | 3.2 ± 0.1 | 15 ± 0.2 |
| 24 | 20.4 ± 0.2 | 5.4 ± 0.2 | 19 ± 0.2 | 19.3 ± 0.3 | 5.4 ± 0.1 | 18 ± 0.4 | 24.7 ± 0.3 | 6.5 ± 0.1 | 23 ± 0.3 | |
| 8 | 12 | 34.6 ± 0.3 | 49.5 ± 0.2 | 36 ± 0.5 | 46.2 ± 0.5 | 54.8 ± 0.3 | 47 ± 0.5 | 51.9 ± 0.5 | 63.4 ± 0.3 | 54 ± 0.5 |
| 24 | 45.0 ± 0.5 | 55.9 ± 0.3 | 46 ± 0.7 | 58.2 ± 0.5 | 66.7 ± 0.3 | 59 ± 0.7 | 70.6 ± 0.6 | 75.3 ± 0.4 | 71 ± 0.7 | |
| 9 | 12 | 48.2 ± 0.5 | 57.0 ± 0.3 | 49 ± 0.6 | 63.7 ± 0.7 | 66.7 ± 0.4 | 64 ± 1.0 | 62.4 ± 0.6 | 68.8 ± 0.3 | 63 ± 0.6 |
| 24 | 67.7 ± 0.7 | 71.0 ± 0.3 | 68 ± 0.9 | 76.7 ± 0.8 | 79.6 ± 0.5 | 77 ± 1.2 | 88.5 ± 0.8 | 88.2 ± 0.5 | 89 ± 1.2 | |
| 10 | 12 | 53.0 ± 0.6 | 63.4 ± 0.2 | 54 ± 0.7 | 69.0 ± 0.7 | 68.8 ± 0.3 | 69 ± 0.8 | 72.5 ± 0.7 | 77.4 ± 0.3 | 73 ± 0.8 |
| 24 | 82.6 ± 0.7 | 87.1 ± 0.3 | 83 ± 1.0 | 91.6 ± 0.8 | 91.4 ± 0.4 | 92 ± 1.4 | 99.4 ± 1.1 | 98.9 ± 0.6 | 98 ± 1.8 | |
| T (°C) | Time (h) | Chitin + CALB | Chitin + VPOSS + CALB | Chitin + MPOSS + CALB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| * | ** | *** | * | ** | *** | * | ** | *** | ||
| 20 | 12 | 21.7 ± 0.2 | 3.2 ± 0.1 | 20 ± 0.3 | 27.7 ± 0.3 | 9.7 ± 0.1 | 26 ± 0.4 | 30.7 ± 0.3 | 34.4 ± 0.2 | 31 ± 0.4 |
| 24 | 42.0 ± 0.4 | 52.7 ± 0.2 | 43 ± 0.6 | 53.4 ± 0.7 | 60.2 ± 0.3 | 54 ± 0.6 | 57.0 ± 0.7 | 67.7 ± 0.2 | 58 ± 0.8 | |
| 30 | 12 | 51.6 ± 0.4 | 55.9 ± 0.2 | 52 ± 0.5 | 60.5 ± 0.7 | 65.6 ± 0.2 | 61 ± 0.5 | 65.6 ± 0.8 | 69.9 ± 0.2 | 66 ± 0.7 |
| 24 | 65.7 ± 0.6 | 68.8 ± 0.1 | 66 ± 0.6 | 80.4 ± 0.7 | 87.1 ± 0.3 | 81 ± 0.8 | 86.5 ± 0.8 | 91.4 ± 0.4 | 87 ± 0.9 | |
| 40 | 12 | 53.0 ± 0.6 | 63.4 ± 0.2 | 54 ± 0.7 | 69.0 ± 0.7 | 68.8 ± 0.3 | 69 ± 0.8 | 72.5 ± 0.7 | 77.4 ± 0.3 | 73 ± 0.8 |
| 24 | 82.6 ± 0.7 | 87.1 ± 0.3 | 83 ± 1.0 | 91.6 ± 0.8 | 95.7 ± 0.4 | 92 ± 1.4 | 99.4 ± 1.1 | 98.9 ± 0.6 | 98 ± 1.8 | |
| 50 | 12 | 47.5 ± 0.5 | 52.7 ± 0.2 | 48 ± 0.7 | 62.4 ± 0.9 | 68.8 ± 0.2 | 63 ± 0.7 | 74.3 ± 0.6 | 81.7 ± 0.4 | 75 ± 0.7 |
| 24 | 69.5 ± 0.7 | 75.3 ± 0.2 | 70 ± 0.9 | 83.8 ± 1.2 | 86.0 ± 0.3 | 84 ± 1.1 | 90.5 ± 1.1 | 95.7 ± 0.4 | 91 ± 1.4 | |
| 60 | 12 | 68.6 ± 0.6 | 73.1 ± 0.2 | 69 ± 0.8 | 72.5 ± 0.6 | 77.4 ± 0.2 | 73 ± 1.0 | 82.6 ± 0.7 | 87.1 ± 0.3 | 83 ± 0.9 |
| 24 | 72.4 ± 0.8 | 89.2 ± 0.2 | 74 ± 1.1 | 77.6 ± 0.8 | 88.2 ± 0.4 | 79 ± 1.2 | 88.1 ± 1.6 | 90.3 ± 0.7 | 87 ± 2.8 | |
| Time (h) | Chitin + CALB | Chitin + VPOSS + CALB | Chitin + MPOSS + CALB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| * | ** | *** | * | ** | *** | * | ** | *** | |
| 1 | 3.3 ± 0.1 | 0.0 ± 0.1 | 3 ± 0.1 | 7.6 ± 0.2 | 1.1 ± 0.1 | 7 ± 0.2 | 12.0 ± 0.2 | 1.1 ± 0.1 | 11 ± 0.2 |
| 2 | 10.9 ± 0.1 | 1.1 ± 0.1 | 10 ± 0.2 | 16.3 ± 0.2 | 2.2 ± 0.1 | 15 ± 0.2 | 20.7 ± 0.2 | 2.2 ± 0.1 | 19 ± 0.2 |
| 6 | 31.0 ± 0.3 | 31.2 ± 0.2 | 31 ± 0.4 | 45.4 ± 0.5 | 51.6 ± 0.3 | 46 ± 0.5 | 51.7 ± 0.4 | 54.8 ± 0.2 | 52 ± 0.6 |
| 12 | 53.0 ± 0.6 | 63.4 ± 0.2 | 54 ± 0.7 | 69.0 ± 0.7 | 68.8 ± 0.3 | 69 ± 0.8 | 72.5 ± 0.7 | 77.4 ± 0.3 | 73 ± 0.8 |
| 24 | 82.6 ± 0.7 | 87.1 ± 0.3 | 83 ± 1.0 | 91.6 ± 0.8 | 95.7 ± 0.4 | 92 ± 1.4 | 99.4 ± 1.1 | 98.9 ± 0.6 | 98 ± 1.8 |
| 72 | 99.9 ± 1.4 | 96.8 ± 0.5 | 98 ± 2.3 | 99.8 ± 1.6 | 98.9 ± 0.6 | 98 ± 2.1 | 99.7 ± 1.2 | 97.8 ± 0.7 | 98 ± 1.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdarta, J.; Wysokowski, M.; Norman, M.; Kołodziejczak-Radzimska, A.; Moszyński, D.; Maciejewski, H.; Ehrlich, H.; Jesionowski, T. Candida antarctica Lipase B Immobilized onto Chitin Conjugated with POSS® Compounds: Useful Tool for Rapeseed Oil Conversion. Int. J. Mol. Sci. 2016, 17, 1581. https://doi.org/10.3390/ijms17091581
Zdarta J, Wysokowski M, Norman M, Kołodziejczak-Radzimska A, Moszyński D, Maciejewski H, Ehrlich H, Jesionowski T. Candida antarctica Lipase B Immobilized onto Chitin Conjugated with POSS® Compounds: Useful Tool for Rapeseed Oil Conversion. International Journal of Molecular Sciences. 2016; 17(9):1581. https://doi.org/10.3390/ijms17091581
Chicago/Turabian StyleZdarta, Jakub, Marcin Wysokowski, Małgorzata Norman, Agnieszka Kołodziejczak-Radzimska, Dariusz Moszyński, Hieronim Maciejewski, Hermann Ehrlich, and Teofil Jesionowski. 2016. "Candida antarctica Lipase B Immobilized onto Chitin Conjugated with POSS® Compounds: Useful Tool for Rapeseed Oil Conversion" International Journal of Molecular Sciences 17, no. 9: 1581. https://doi.org/10.3390/ijms17091581
APA StyleZdarta, J., Wysokowski, M., Norman, M., Kołodziejczak-Radzimska, A., Moszyński, D., Maciejewski, H., Ehrlich, H., & Jesionowski, T. (2016). Candida antarctica Lipase B Immobilized onto Chitin Conjugated with POSS® Compounds: Useful Tool for Rapeseed Oil Conversion. International Journal of Molecular Sciences, 17(9), 1581. https://doi.org/10.3390/ijms17091581