Sodium Copper Chlorophyllin Immobilization onto Hippospongia communis Marine Demosponge Skeleton and Its Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adsorption Tests
2.1.1. Effect of Contact Time
2.1.2. Effect of Initial Dye Concentration
2.1.3. Effect of pH
2.1.4. Effect of Ionic Strength
2.2. Kinetic and Isothermal Studies
2.3. Desorption Test
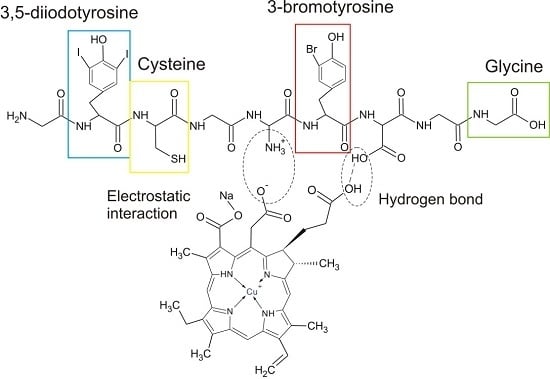
2.4. Structural Analysis
2.5. Antibacterial Tests
3. Materials and Methods
3.1. Materials
3.2. Adsorption and Desorption Tests
3.3. Analysis
3.4. Antibacterial Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hou, X.; Yang, R.; Xu, H.; Yang, Y. Adsorption kinetic and thermodynamic studies of silk dyed with sodium copper chlorophyllin. Ind. Eng. Chem. Res. 2012, 51, 8341–8347. [Google Scholar] [CrossRef]
- Mortensen, A.; Geppel, A. HPLC–MS analysis of the green food colorant sodium copper chlorophyllin. Innov. Food Sci. Emerg. Technol. 2007, 8, 419–425. [Google Scholar] [CrossRef]
- Dashwood, R.H. The importance of using pure chemicals in (anti) mutagenicity studies: Chlorophyllin as a case in point. Mutat. Res. 1997, 381, 283–286. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Failla, M.L.; Schwartz, S.J. Sodium copper chlorophyllin: In vitro digestive stability and accumulation by caco-2 human intestinal cells. J. Agric. Food. Chem. 2002, 50, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Kim, J.H.; Shin, B.A.; Lee, H.C.; Kim, Y.S.; Lim, H.S.; Oh, J.S. Inhibitory effect of chlorophyllin on the Propionibacterium acnes-induced chemokine expression. J. Microbiol. 2013, 51, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chu, J. Preparation and in-vitro release performance of sodium-iron chlorophyllin microcapsules. Procedia Environ. Sci. 2011, 8, 270–275. [Google Scholar] [CrossRef]
- Toyoda, T.; Cho, Y.M.; Mizuta, Y.; Akagi, J.I.; Ogawa, K. A 13-week subchronic toxicity study of ferric citrate in F344 rats. Food Chem. Toxicol. 2014, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Zhang, L.; Wang, Y.; Jiang, H.; Ren, Y. Fe-chlorophyllin promotes the growth of wheat roots associated with nitric oxide generation. Int. J. Mol. Sci. 2010, 11, 5246–5255. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Y.; Gao, J.; Jin, X.; Wang, Z.; Wang, B.; Li, K.; Li, Y. Detection and comparison of reactive oxygen species (ROS) generated by chlorophyllin metal (Fe, Mg and Cu) complexes under ultrasonic and visible-light irradiation. Ultrason. Sonochem. 2011, 18, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandtt, P.; Spiro, T.G. Surface-enhanced resonance Raman spectroscopy of copper chlorophyllin on silver and gold colloids. J. Phys. Chem. 1988, 92, 3355–3360. [Google Scholar] [CrossRef]
- Chernomorsky, S.; Segelman, A.; Poretz, R.D. Effect of dietary chlorophyll derivatives on mutagenesis and tumor cell growth. Teratog. Carcinog. Mutagen. 1999, 19, 313–322. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Peñaloza, E.P.; Cruces Martínez, M.P. Sodium copper chlorophyllin (SCC) induces genetic damage in postmeiotic and somatic wing cells of Drosophila melanogaster. J. Toxicol. Environ. Health 2013, 76, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Hayatsu, H.; Sugiyama, C.; Arimoto-Kobayashi, S.; Negishi, T. Porphyrins as possible preventers of heterocyclic amine carcinogenesis. Cancer Lett. 1999, 143, 185–187. [Google Scholar] [CrossRef]
- Sugiyama, C.; Nakandakari, N.; Hayatsu, H.; Arimoto-Kobayashi, S. Preventive effects of chlorophyllin fixed on chitosan towards DNA adduct formation of 3-amino-1-methyl-5 H-pyrido [4,3-b] indole in CDF 1 mice. Biol. Pharm. Bull. 2002, 25, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Aozasa, O.; Tetsumi, T.; Ohta, S.; Nakao, T.; Miyata, H.; Nomura, T. Fecal excretion of dioxin in mice enhanced by intake of dietary fiber bearing chlorophyllin. Bull. Environ. Contam. Toxicol. 2003, 70, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, V.; Schimerlik, M.; Dashwood, R.; Bailey, G. Mechanisms of chlorophyllin anticarcinogenesis against Aflatoxin B: Complex formation with the carcinogen. Chem. Res. Toxicol. 1995, 8, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Egner, P.A.; Wang, J.; Zhu, Y.; Zhang, B.; Wu, Y.; Zhang, Q.; Qian, G.S.; Kuang, S.Y.; Gange, S.J.; Jacobson, L.P.; et al. Chlorophyllin intervention reduces aflatoxin—DNA adducts in individuals at high risk for liver cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14601–14606. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, S.; Kanyama, K.; Rai, H.; Hayatsu, H. Inhibitory effect of hemin, chlorophyllin and related pyrrole pigments on the mutagenicity of benzo[a]pyrene and its metabolites. Mutat. Res. 1995, 345, 127–135. [Google Scholar] [CrossRef]
- Chernomorsky, S.; Rancourt, R.; Virdi, K. Antimutagenicity, cytotoxicity and composition of chlorophyllin copper complex. Cancer Lett. 1997, 120, 141–147. [Google Scholar] [CrossRef]
- Neault, J.F.; Tajmir-Riahi, H.A. DNA—Chlorophyllin interaction. J. Phys. Chem. B 1998, 102, 1610–1614. [Google Scholar] [CrossRef]
- Tumolo, T.; Lanfer-Marquez, U.M. Copper chlorophyllin: A food colorant with bioactive properties? Food Res. Int. 2012, 46, 451–459. [Google Scholar] [CrossRef]
- Gomes, B.B.; Barros, S.B.; Andrade-Wartha, E.R.; Silva, A.M.; Silva, V.V.; Lanfer-Marquez, U.M. Bioavailability of dietary sodium copper chlorophyllin and its effect on antioxidant defence parameters of Wistar rats. J. Sci. Food Agric. 2009, 89, 2003–2010. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar]
- Luksiene, Z.; Buchovec, I.; Paskeviciute, E. Inactivation of Bacillus cereus by Na-chlorophyllin-based photosensitization on the surface of packaging. J. Appl. Microbiol. 2010, 109, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Luksiene, Z.; Paskeviciute, E. Microbial control of food-related surfaces: Na-chlorophyllin-based photosensitization. J. Photochem. Photobiol. 2011, 105, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Hendry, G.A.F.; Houghton, J.D. Natural Food Colorants, 2nd ed.; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Park, S.J.; Park, Y.M. Eco-dyeing and antimicrobial properties of chlorophyllin copper complex extracted from Sasa veitchii fiber. Polymer 2010, 11, 357–362. [Google Scholar]
- Yamazaki, H.; Fujieda, M.; Togashi, M.; Saito, T.; Preti, G.; Cashman, J.R.; Kamataki, T. Effects of the dietary supplements, activated charcoal and copper chlorophyllin, on urinary excretion of trimethylamine in Japanese trimethylaminuria patients. Life Sci. 2004, 74, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Falabella, A.F. Debridement and wound bed preparation. Dermatol. Ther. 2006, 19, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.; Grätzel, M. Artificial photosynthesis. 1. Photosensitization of TiO2 solar cells with chlorophyll derivatives and related natural porphyrins. J. Phys. Chem. 1993, 97, 6272–6277. [Google Scholar] [CrossRef]
- Arimoto-Kobayashi, S.; Harada, N.; Tokunaga, R.; Odo, J. Adsorption of mutagens to chlorophyllin—Chitosan, an insoluble form of chlorophyllin. Mutat. Res. 1997, 381, 243–249. [Google Scholar] [CrossRef]
- Oliveira, G.R.; do Amaral, L.J.; Giovanela, M.; da Silva Crespo, J.; Fetter, G.; Rivera, J.A.; Sampieri, A.; Bosch, P. Bactericidal performance of chlorophyllin-copper hydrotalcite compounds. Water Air Soil. Pollut. 2015, 226, 1–12. [Google Scholar] [CrossRef]
- Azimi, S.; Behin, J.; Abiri, R.; Rajabi, L.; Derakhshan, A.A.; Karimnezhad, S.H. Synthesis, characterization and antibacterial activity of chlorophyllin functionalized graphene oxide nanostructures. Sci. Adv. Mater. 2014, 6, 771–781. [Google Scholar] [CrossRef]
- Wen, W.; Wan, J.; Cao, X.; Xia, J. Preparation of a light-sensitive and reversible dissolution copolymer and its application in lysozyme purification. Biotechnol. Prog. 2007, 23, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Schleuter, D.; Günther, A.; Paasch, S.; Ehrlich, H.; Kljajić, Z.; Hanke, T.; Bernhardt, G.; Brunner, E. Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohyd. Polym. 2013, 92, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Bartczak, P.; Zdarta, J.; Tylus, W.; Szatkowski, T.; Stelling, A.L.; Ehrlich, H.; Jesionowski, T. Adsorption of C.I. Natural Red 4 onto spongin skeleton of marine Demosponge. Materials 2015, 8, 96–116. [Google Scholar] [CrossRef]
- Green, D.; Walsh, D.; Mann, S.; Oreffo, R.O.C. The potential of biomimesis in bone tissue engineering: Lessons from the design and synthesis of invertebrate skeletons. Bone 2002, 30, 810–815. [Google Scholar] [CrossRef]
- Green, D. Tissue bionics: Examples in biomimetic tissue engineering. Biomed. Mater. 2008, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O.C. Natural marine sponge fiber skeleton: A biomimetic scaffold for human osteoprogenitor cell attachment, growth, and differentiation. Tissue Eng. 2003, 9, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Worch, H. Sponges as natural composites: From biomimetic potential to development of new biomaterials. In Porifera Research-Biodiversity, Innovation & Sustainability; Custodio, M.R., Lobo-Hajdu, G., Hajdu, E., Muricy, G., Eds.; Museu Nacional: Rio de Janeiro, Brazil, 2009; pp. 303–312. [Google Scholar]
- Szatkowski, T.; Wysokowski, M.; Lota, G.; Pęziak, D.; Bazhenov, V.V.; Nowaczyk, G.; Walter, J.; Molodtsov, S.L.; Stocker, H.; Himcinschi, C.; et al. Novel nanostructured hematite–spongin composite developed using an extreme biomimetic approach. RSC Adv. 2015, 5, 79031–79040. [Google Scholar] [CrossRef]
- Heaton, J.W.; Marangoni, A.G. Chlorophyll degradation in processed foods and senescent plant tissues. Trends Food Sci. Technol. 1996, 7, 8–15. [Google Scholar] [CrossRef]
- Gómez, J.M.; Galán, J.; Rodríguez, A.; Walker, G.M. Dye adsorption onto mesoporous materials: pH influence, kinetics and equilibrium in buffered and saline media. J. Environ. Manag. 2014, 146, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, X.; Tang, H.; Su, Y. Sorption behavior of dye compounds onto natural sediment of Qinghe River. J. Colloid Interface Sci. 2001, 239, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Juang, R.S. Equilibrium and kinetic studies on the adsorption of surfactant, organic acids and dyes from water onto natural biopolymers. Colloids Surf. A 2005, 269, 35–46. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Gimbert, F.; Morin-Crini, N.; Renault, F.; Badot, P.M.; Crini, G. Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: Error analysis. J. Hazard. Mater. 2008, 157, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Farag, A.A.M.; Mansour, A.M.; Ammar, A.H.; Abdel Rafea, M.; Farid, A.M. Electrical conductivity, dielectric properties and optical absorption of organic based nanocrystalline sodium copper chlorophyllin for photodiode application. J. Alloys Compd. 2012, 513, 404–413. [Google Scholar] [CrossRef]
- Marty, R.; Ouameur, A.A.; Neault, J.F.; Tajmir-Riahi, H.A. RNA adducts with chlorophyll and chlorophyllin: Stability and structural features. J. Biomol. Struct. Dyn. 2004, 22, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Biological Materials of Marine Origin. Invertebrates, 1st ed.; Springer Science + Business Media: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Araújo, M.F.; Cruz, A.; Humanes, M.; Lopes, M.T.; Da Silva, J.A.L.; Da Silva, J.J.R.F. Elemental composition of Demospongiae from the eastern Atlantic coastal waters. Chem. Speciat. Bioavailab. 1999, 11, 25–36. [Google Scholar] [CrossRef]
- Hynninen, P.H.; Leppäkases, T.S.; Mesilaakso, M. The enolate anions of chlorophylls a and b as ambident nucleophiles in oxidations with (−)- or (+)-(10-camphorsulfonyl)oxaziridine. Synthesis of 132(S/R)-hydroxychlorophylls a and b. Tetrahedron 2006, 62, 3412–3422. [Google Scholar] [CrossRef]
- Willows, R.D.; Li, Y.; Scheer, H.; Chen, M. Structure of chlorophyll F. Org. Lett. 2013, 15, 1588–1590. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.H.A.; Carvalhoda-Silva, D.; Santos, J.L.M.S.; Weitner, T.; Fonseca, M.G.; Yoshida, M.I.; Idemori, Y.M.; Batinic-Haberlec, I.; Reboucas, J.S. Thermal stability of the prototypical Mn porphyrin-based superoxide dismutase mimic and potent oxidative-stress redox modulator Mn(III) meso-tetrakis (N-ethylpyridinium-2-yl)porphyrin chloride, MnTE-2-PyP(5+). J. Pharm. Biomed. Anal. 2013, 73, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Gokakakar, S.D.; Salker, A.V. Synthesis, purification and thermal behaviour of sulfonated metalloporphyrins. J. Therm. Anal. Calorim. 2011, 109, 1487–1492. [Google Scholar] [CrossRef]
- Velićanski, A.S.; Cvetković, D.D.; Markov, S.L.; Vulić, J.J.; Djilas, S.M. Antibacterial activity of β vulgaris L. pomace extract. Acta Period. Technol. 2011, 42, 263–269. [Google Scholar] [CrossRef]
- Almeida Alves, T.M.; Silva, A.F.; Brandão, M.; Grandi, T.S.M.; Smania, E.F.; Júnior, A.S.; Zani, C.L. Biological screening of brazilian medicinal plants. Mem. Inst. Oswaldo Cruz 2000, 95, 367–373. [Google Scholar] [CrossRef]
- Rifai, S.; Fassouane, A.; El-Abbouyi, A.; Wardani, A.; Kijjoa, A.; van Soest, R. Screening of antimicrobial activity of marine sponge extracts. J. Mycol. Med. 2005, 15, 33–38. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Huanga, K.C.; Mukhopadhyayb, R.; Wena, W.; Gitaia, Z.; Wingreen, N.S. Cell shape and cell-wall organization in Gram-negative bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 19282–19287. [Google Scholar] [CrossRef] [PubMed]










| Dye Concentration (mg/L) | Ionic Strength (mol/L NaCl) | pH = 5 | pH = 7 | pH = 11 | |||
|---|---|---|---|---|---|---|---|
| qt (mg/g) | E (%) | qt (mg/g) | E (%) | qt (mg/g) | E (%) | ||
| 25 | - | 6.25 | 100 | 5.58 | 89.24 | 0.25 | 3.95 |
| 50 | 12.50 | 100 | 3.78 | 30.24 | 1.32 | 10.58 | |
| 100 | 24.96 | 99.85 | 5.49 | 21.96 | 2.72 | 10.87 | |
| 200 | 49.35 | 98.70 | 6.82 | 13.64 | 5.60 | 11.20 | |
| 25 | 0.01 | 6.25 | 100 | 4.61 | 73.72 | 1.00 | 15.97 |
| 50 | 12.50 | 100 | 3.17 | 25.37 | 0.44 | 3.49 | |
| 100 | 25.00 | 100 | 4.67 | 18.67 | 1.01 | 4.04 | |
| 200 | 49.96 | 99.93 | 6.35 | 12.70 | 2.90 | 5.81 | |
| 25 | 0.1 | 6.25 | 100 | 5.99 | 95.86 | 2.50 | 40.08 |
| 50 | 12.50 | 100 | 12.34 | 98.72 | 3.12 | 24.92 | |
| 100 | 25.00 | 100 | 24.57 | 98.29 | 4.75 | 19.00 | |
| 200 | 50.00 | 100 | 49.73 | 99.46 | 6.97 | 13.94 | |
| Kinetic Models | Parameters | Initial Dye Concentration | ||
|---|---|---|---|---|
| 100 (mg/L) | 200 (mg/L) | 300 (mg/L) | ||
| Pseudo-First Order | qe,exp (mg/g) | 24.84 | 49.73 | 74.57 |
| qe,cal (mg/g) | 0.17 | 14.62 | 12.80 | |
| k1 (1/min) | 0.006 | 0.084 | 0.076 | |
| r2 | 0.003 | 0.327 | 0.404 | |
| Pseudo-Second Order | qe,cal (mg/g) | 24.87 | 49.83 | 75.85 |
| k2 (g/mg·min) | 0.102 | 0.010 | 0.009 | |
| r2 | 0.999 | 0.997 | 0.999 | |
| h | 63.07 | 24.80 | 50.94 | |
| Element | Content of Elements by Weight (%) | |||
|---|---|---|---|---|
| Chlorophyllin | Sponge Skeleton (SS) | SS + SCC (400 mg/L) | SS + SCC (1000 mg/L) | |
| C | 84.66 | 81.67 | 88.13 | 82.85 |
| N | 4.48 | 3.18 | 0.20 | 2.56 |
| O | 4.84 | 10.37 | 2.62 | 8.08 |
| Cu | 2.80 | - | 0.26 | 0.53 |
| Cl | 0.42 | 0.34 | 5.45 | 1.52 |
| Na | 2.79 | - | 1.91 | 0.80 |
| Al | - | 1.17 | 0.27 | 0.77 |
| Si | - | 0.25 | 0.04 | 0.10 |
| S | - | 1.15 | 0.31 | 0.87 |
| I | - | 1.87 | 0.82 | 1.92 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norman, M.; Bartczak, P.; Zdarta, J.; Tomala, W.; Żurańska, B.; Dobrowolska, A.; Piasecki, A.; Czaczyk, K.; Ehrlich, H.; Jesionowski, T. Sodium Copper Chlorophyllin Immobilization onto Hippospongia communis Marine Demosponge Skeleton and Its Antibacterial Activity. Int. J. Mol. Sci. 2016, 17, 1564. https://doi.org/10.3390/ijms17101564
Norman M, Bartczak P, Zdarta J, Tomala W, Żurańska B, Dobrowolska A, Piasecki A, Czaczyk K, Ehrlich H, Jesionowski T. Sodium Copper Chlorophyllin Immobilization onto Hippospongia communis Marine Demosponge Skeleton and Its Antibacterial Activity. International Journal of Molecular Sciences. 2016; 17(10):1564. https://doi.org/10.3390/ijms17101564
Chicago/Turabian StyleNorman, Małgorzata, Przemysław Bartczak, Jakub Zdarta, Wiktor Tomala, Barbara Żurańska, Anna Dobrowolska, Adam Piasecki, Katarzyna Czaczyk, Hermann Ehrlich, and Teofil Jesionowski. 2016. "Sodium Copper Chlorophyllin Immobilization onto Hippospongia communis Marine Demosponge Skeleton and Its Antibacterial Activity" International Journal of Molecular Sciences 17, no. 10: 1564. https://doi.org/10.3390/ijms17101564
APA StyleNorman, M., Bartczak, P., Zdarta, J., Tomala, W., Żurańska, B., Dobrowolska, A., Piasecki, A., Czaczyk, K., Ehrlich, H., & Jesionowski, T. (2016). Sodium Copper Chlorophyllin Immobilization onto Hippospongia communis Marine Demosponge Skeleton and Its Antibacterial Activity. International Journal of Molecular Sciences, 17(10), 1564. https://doi.org/10.3390/ijms17101564