McDonald Criteria 2010 and 2005 Compared: Persistence of High Oligoclonal Band Prevalence Despite Almost Doubled Diagnostic Sensitivity
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics in Patients with MS and CIS
2.2. CSF Changes in MS Patients
2.3. CSF Changes in CIS Patients
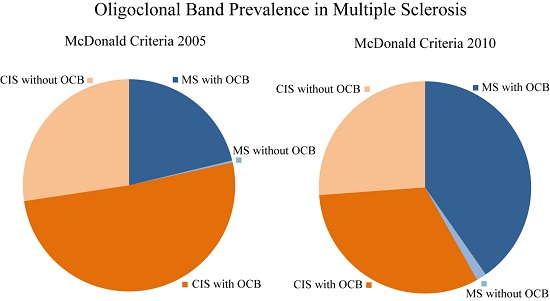
2.4. Comparison Between the Different McDonald Criteria
2.5. Subgroup Analysis of CSF of MS Patients Diagnosed according to the McDonald Criteria of 2010
3. Discussion
4. Methods
4.1. Patients
4.2. CSF and Serum Analytical Procedures
4.3. Magnetic Resonance Imaging (MRI)
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, D.H.; Chard, D.T.; Ciccarelli, O. Clinically isolated syndromes. Lancet Neurol. 2012, 11, 157–169. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2002, 359, 1221–1231. [Google Scholar] [CrossRef]
- Comi, G.; Filippi, M.; Barkhof, F.; Durelli, L.; Edan, G.; Fernandez, O.; Hartung, H.P.; Seeldrayers, P.; Sørensen, P.S.; Rovaris, M.; et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: A randomised study. Lancet 2001, 357, 1576–1582. [Google Scholar] [CrossRef]
- Comi, G.; Martinelli, V.; Rodegher, M.; Moiola, L.; Bajenaru, O.; Carra, A.; Elovaara, I.; Fazekas, F.; Hartung, H.P.; Hillert, J.; et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): A randomised, double-blind, placebo-controlled trial. Lancet 2009, 374, 1503–1511. [Google Scholar] [CrossRef]
- Jacobs, L.D.; Beck, R.W.; Simon, J.H.; Kinkel, R.P.; Brownscheidle, C.M.; Murray, T.J.; Simonian, N.A.; Slasor, P.J.; Sandrock, A.W. Intramuscular interferon β-1a therapy initiated during a first demyelinating event in multiple sclerosis. N. Engl. J. Med. 2000, 343, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Polman, C.H.; Freedman, M.S.; Edan, G.; Hartung, H.P.; Miller, D.H.; Montalban, X.; Barkhof, F.; Bauer, L.; Jakobs, P.; et al. Treatment with interferon β-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006, 67, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Stangel, M.; Fredrikson, S.; Meinl, E.; Petzold, A.; Stuve, O.; Tumani, H. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat. Rev. Neurol. 2013, 9, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Tumani, H.; Hartung, H.P.; Hemmer, B.; Teunissen, C.; Deisenhammer, F.; Giovannoni, G.; Zettl, U.K.; BioMS Study Group. Cerebrospinal fluid biomarkers in multiple sclerosis. Neurobiol. Dis. 2009, 35, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Link, H.; Huang, Y.M. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: An update on methodology and clinical usefulness. J. Neuroimmunol. 2006, 180, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, J.; Disanto, G.; Dobson, R.; Adiutori, R.; Bianchi, L.; Topping, J.; Bestwick, J.P.; Meier, U.C.; Marta, M.; Dalla Costa, G.; et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult. Scler. 2015, 21, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Bosca, I.; Magraner, M.J.; Coret, F.; Alvarez-Cermeno, J.C.; Simo-Castello, M.; Villar, L.M.; Casanova, B. The risk of relapse after a clinically isolated syndrome is related to the pattern of oligoclonal bands. J. Neuroimmunol. 2010, 226, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Joseph, F.G.; Hirst, C.L.; Pickersgill, T.P.; Ben-Shlomo, Y.; Robertson, N.P.; Scolding, N.J. CSF oligoclonal band status informs prognosis in multiple sclerosis: A case control study of 100 patients. J. Neurol. Neurosurg. Psychiatry 2009, 80, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Ramagopalan, S.; Davis, A.; Giovannoni, G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: A meta-analysis of prevalence, prognosis and effect of latitude. J. Neurol. Neurosurg. Psychiatry 2013, 84, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.S.; Thompson, E.J.; Deisenhammer, F.; Giovannoni, G.; Grimsley, G.; Keir, G.; Öhman, S.; Racke, M.K.; Sharief, M.; Sindic, C.J.; et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch. Neurol. 2005, 62, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A. Intrathecal oligoclonal IgG synthesis in multiple sclerosis. J. Neuroimmunol. 2013, 262, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zeman, A.Z.; Kidd, D.; McLean, B.N.; Kelly, M.A.; Francis, D.A.; Miller, D.H.; Kendall, B.E.; Rudge, P.; Thompson, E.J.; McDonald, W.I. A study of oligoclonal band negative multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1996, 60, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Keir, G.; Thompson, E.J.; Giovannoni, G. The clinical significance of an intrathecal monoclonal immunoglobulin band: A follow-up study. Neurology 2003, 60, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Tintore, M.; Rovira, A.; Rio, J.; Tur, C.; Pelayo, R.; Nos, C.; Tellez, N.; Perkal, H.; Comabella, M.; Sastre-Garriga, J.; et al. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology 2008, 70, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Tintore, M.; Rovira, A.; Rio, J.; Otero-Romero, S.; Arrambide, G.; Tur, C.; Comabella, M.; Nos, C.; Arévalo, M.J.; Negrotto, L.; et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 2015, 138, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Skripuletz, T.; Schwenkenbecher, P.; Pars, K.; Stoll, M.; Conzen, J.; Bolat, S.; Pul, R.; Vonberg, R.P.; Sedlacek, L.; Wurster, U.; et al. Importance of follow-up cerebrospinal fluid analysis in cryptococcal meningoencephalitis. Dis. Markers 2014, 2014, 162576. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H. Cerebrospinal fluid-physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult. Scler. 1998, 4, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Alvarez-Cermeno, J.; Bernardi, G.; Cogato, I.; Fredman, P.; Frederiksen, J.; Fredrikson, S.; Gallo, P.; Grimaldi, L.M.; Grønning, M.; et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: A consensus report. J. Neurol. Neurosurg. Psychiatry 1994, 57, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H. External quality assessment in clinical neurochemistry: Survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin. Chem. 1995, 41, 256–263. [Google Scholar] [PubMed]
- Swanton, J.K.; Rovira, A.; Tintore, M.; Altmann, D.R.; Barkhof, F.; Filippi, M.; Huerga, E.; Miszkiel, K.A.; Plant, G.T.; Polman, C.; et al. MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: A multicentre retrospective study. Lancet Neurol. 2007, 6, 677–686. [Google Scholar] [CrossRef]
- Tintore, M.; Rovira, A.; Martinez, M.J.; Rio, J.; Diaz-Villoslada, P.; Brieva, L.; Borrás, C.; Grivé, E.; Capellades, J.; Montalban, X. Isolated demyelinating syndromes: Comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. Am. J. Neuroradiol. 2000, 21, 702–706. [Google Scholar] [PubMed]

| Characteristics (McDonald 2010) | Patient Numbers | Pleocytosis (≥5 cells/µL) | Lactate (>3.5 mmol/L) | Protein (>500/mg/L) | Blood-CSF-Barrier Dysfunction | Intrathecal Synthesis | CSF Oligoclonal Bands | ||
|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | IgA | |||||||
| Multiple Sclerosis | 136 | 65% | 1% | 28% | 26% | 35% | 63% | 13% | 96% |
| Optic neuritis | 44 | 66% | 0% | 25% | 18% | 32% | 64% | 11% | 98% |
| Paresis/Sensory symptoms | 30 | 63% | 0% | 27% | 30% | 30% | 73% | 20% | 97% |
| Brainstem symptoms | 19 | 79% | 0% | 26% | 21% | 37% | 47% | 16% | 95% |
| Spinal cord symptoms | 30 | 57% | 0% | 33% | 37% | 40% | 57% | 7% | 93% |
| Polysymptomatic | 13 | 69% | 8% | 31% | 31% | 46% | 69% | 15% | 100% |
| Clinically Isolated Syndrome | 189 | 52% | 0% | 21% | 22% | 14% | 33% | 4% | 55% |
| Optic neuritis | 136 | 50% | 0% | 18% | 23% | 13% | 23% | 3% | 46% |
| Paresis/Sensory symptoms | 11 | 45% | 0% | 27% | 9% | 27% | 45% | 27% | 55% |
| Brainstem symptoms | 16 | 44% | 0% | 19% | 13% | 19% | 63% | 0% | 88% |
| Spinal cord symptoms | 26 | 69% | 0% | 35% | 31% | 15% | 62% | 4% | 85% |
| p value | - | p = 0.1 | p = 1.0 | p = 0.3 | p = 0.6 | p = 0.0009 | p = 0.0001 | p = 0.04 | p = 0.0001 |
| Combinations of Antibody Classes | Multiple Sclerosis | Clinically Isolated Syndrome | ||
|---|---|---|---|---|
| Intrathecal Synthesis of Immunoglobulins | ||||
| Reiber-Felgenhauer | Reiber-Felgenhauer + OCB | Reiber-Felgenhauer | Reiber-Felgenhauer + OCB | |
| 3-class synthesis of IgG + IgM + IgA | 7% | 8% | 3% | 3% |
| 2-class synthesis of IgG + IgM | 21% | 27% | 11% | 11% |
| 2-class synthesis of IgG + IgA | 4% | 5% | 1% | 1% |
| 2-class synthesis of IgM + IgA | 1% | 0 | 1% | 0 |
| Isolated synthesis of IgG | 29% | 54% | 19% | 41% |
| Isolated synthesis of IgM | 5% | 0 | 1% | 0 |
| Isolated synthesis of IgA | 1% | 0 | 0 | 0 |
| Classification | McDonald Criteria | McDonald Criteria | Pleocytosis (≥5 cells/µL) | Intrathecal Synthesis | CSF Oligoclonal Bands | ||
|---|---|---|---|---|---|---|---|
| IgM | IgG | IgA | |||||
| Multiple sclerosis | 2005/2001 | 70 | 66% | 37% | 64% | 16% | 98.5% |
| 2010 | 136 | 65% | 35% | 63% | 13% | 96% | |
| p value | - | p = 0.0001 | p = 1.0 | p = 0.9 | p = 1.0 | p = 0.7 | p = 0.4 |
| Clinically isolated syndrome | 2005/2001 | 255 | 55% | 19% | 40% | 6% | 65% |
| 2010 | 189 | 52% | 14% | 33% | 4% | 55% | |
| p value | - | p = 0.0001 | p = 0.8 | p = 0.4 | p = 0.4 | p = 0.7 | p = 0.2 |
| Multiple Sclerosis (McDonald 2010) Subgroups | Patient Numbers | Pleocytosis (≥5 cells/µL) | Intrathecal Synthesis | CSF Oligoclonal Bands | ||
|---|---|---|---|---|---|---|
| IgM | IgG | IgA | ||||
| 1 relapse and MRI dissemination in space and time | 60 | 72% | 35% | 65% | 12% | 100% |
| ≥2 relapses and MRI dissemination in space and time | 39 | 56% | 36% | 59% | 15% | 97% |
| ≥2 relapses and MRI dissemination in space only | 37 | 65% | 32% | 62% | 14% | 89% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwenkenbecher, P.; Sarikidi, A.; Wurster, U.; Bronzlik, P.; Sühs, K.-W.; Raab, P.; Stangel, M.; Pul, R.; Skripuletz, T. McDonald Criteria 2010 and 2005 Compared: Persistence of High Oligoclonal Band Prevalence Despite Almost Doubled Diagnostic Sensitivity. Int. J. Mol. Sci. 2016, 17, 1592. https://doi.org/10.3390/ijms17091592
Schwenkenbecher P, Sarikidi A, Wurster U, Bronzlik P, Sühs K-W, Raab P, Stangel M, Pul R, Skripuletz T. McDonald Criteria 2010 and 2005 Compared: Persistence of High Oligoclonal Band Prevalence Despite Almost Doubled Diagnostic Sensitivity. International Journal of Molecular Sciences. 2016; 17(9):1592. https://doi.org/10.3390/ijms17091592
Chicago/Turabian StyleSchwenkenbecher, Philipp, Anastasia Sarikidi, Ulrich Wurster, Paul Bronzlik, Kurt-Wolfram Sühs, Peter Raab, Martin Stangel, Refik Pul, and Thomas Skripuletz. 2016. "McDonald Criteria 2010 and 2005 Compared: Persistence of High Oligoclonal Band Prevalence Despite Almost Doubled Diagnostic Sensitivity" International Journal of Molecular Sciences 17, no. 9: 1592. https://doi.org/10.3390/ijms17091592
APA StyleSchwenkenbecher, P., Sarikidi, A., Wurster, U., Bronzlik, P., Sühs, K. -W., Raab, P., Stangel, M., Pul, R., & Skripuletz, T. (2016). McDonald Criteria 2010 and 2005 Compared: Persistence of High Oligoclonal Band Prevalence Despite Almost Doubled Diagnostic Sensitivity. International Journal of Molecular Sciences, 17(9), 1592. https://doi.org/10.3390/ijms17091592