Antidiabetic, Lipid Normalizing, and Nephroprotective Actions of the Strawberry: A Potent Supplementary Fruit
Abstract
:1. Introduction
2. Results
2.1. Animal Observations
2.2. Strawberry Extracts Modulate Serum Glucose Levels
2.3. Strawberry Extracts Improve Liver Function
2.4. Strawberry Extracts Effectively Regulate the Lipid Profile
2.5. Serum Creatinine Levels
2.6. Antioxidant Markers from Liver
2.7. Strawberry Interventions Decreased MDA Content
2.8. Strawberry Interventions Increased Catalase Activity in the Liver
2.9. qRT-PCR Analysis of Fatty Acid Metabolism Genes, Inflammatory Markers, and Their Transcription Factors fromthe Liver
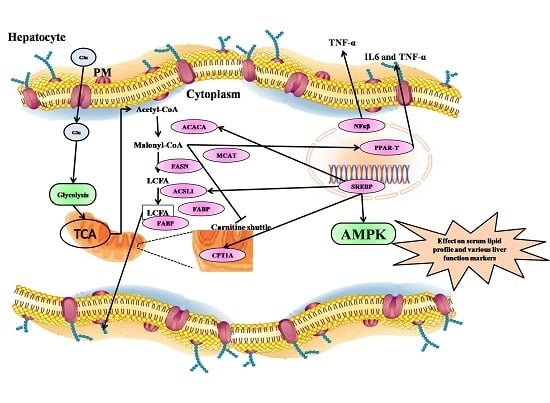
2.10. Fatty Acid Metabolism Genes Were Regulated by Treatment with Strawberry Extracts
2.11. Transcription Factors Expression Reverts Back to Near Normal after Intervention with Strawberry Extract
2.12. Inflammatory Markers Were Effectively Downregulated by Strawberry Extract Interventions
2.13. Histology Examination of Liver, Pancreas, Kidney, and Brain Tissue
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Collection of Strawberry Fruit
4.3. Preparation of Extract
4.4. Experimental Animals
4.5. Experimental Induction of Diabetes
4.6. Collection of Blood and Tissues
4.7. Blood Biochemistry from Serum
4.8. Antioxidant Enzyme Assays from Liver
4.9. Protein Estimation
4.10. Estimation of Malondialdehyde (MDA)
4.11. Estimation of Catalase (CAT)
4.12. Expression Study from Liver cDNA
4.13. Histological Examination and Estimation of Staining Intensity
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Georg, P.; Ludvik, B. Lipids and diabetes. J. Clin. Basic Cardiol. 2000, 3, 159–162. [Google Scholar]
- Nyholm, B.; Pørksen, N.; Juhl, C.B.; Gravholt, C.H.; Butler, P.C.; Weeke, J.; Veldhuis, J.D.; Pincus, S.; Schmitz, O. Assessment of insulin secretion in relatives of patients with type2 (non-insulin-dependent) diabetes mellitus: Evidence of early β-cell dysfunction. Metabolism 2000, 49, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S. The insulin resistance epidemic in India: Fetal origins, later lifestyle, or both? Nutr. Rev. 2001, 59, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Ramarao, P. Animal models in type 2 diabetes research: An overview. Indian J. Med. Res. 2007, 125, 451–472. [Google Scholar] [PubMed]
- Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; Mooradian, A.D.; et al. Nutrition recommendations and interventions for diabetes: A position statement of the american diabetes association. Diabetes Care 2008, 31, 61–78. [Google Scholar]
- Franz, M.J.; Powers, M.A.; Leontos, C.; Holzmeister, L.A.; Kulkarni, K.; Monk, A.; Wedel, N.; Gradwell, E. The evidence for medical nutrition therapy for type 1 and type 2 diabetes in adults. J. Am. Diet. Assoc. 2010, 110, 1852–1889. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.F.; Wylie-Rosett, J. Medical nutrition therapy: A key to diabetes management and prevention. Clin. Diabetes 2010, 28, 12–18. [Google Scholar] [CrossRef]
- Mann, J.I.; de Leeuw, I.; Hermansen, K.; Karamanos, B.; Karlström, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.A.; Rizkalla, S.; Slama, G.; et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Feeney, M.J. Fruits and the prevention of lifestyle-related diseases. Clin. Exp. Pharmacol. Physiol. 2004, 31, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.F.; Macdonald, R.; Lovegrove, J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. Br. J. Nutr. 2010, 104, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S.; Tunon, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Dauchet, L.; Amouyel, P.; Hercberg, S.; Dallongeville, J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 2006, 136, 2588–2593. [Google Scholar] [PubMed]
- Martinez-Gonzalez, M.A.; Lamuela-Raventos, R.M. The unparalleled benefits of fruit. Br. J. Nutr. 2009, 102, 947–948. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Lau, R.; Chan, D.S.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology 2011, 141, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Alvarez-Suarez, J.M.; Busco, F.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry consumption improves plasma antioxidant status and erythrocyte resistance to oxidative haemolysis in humans. Food Chem. 2011, 128, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.L.; Pascual-Teresa, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Identification of anthocyanin pigments in strawberry (cv. Camarosa.) by LC using DAD and ESI-MS detection. Eur. Food Res. Technol. 2002, 214, 248–253. [Google Scholar] [CrossRef]
- Seeram, N.P.; Lee, R.; Scheuller, H.S.; Heber, D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectrometry. Food Chem. 2006, 97, 1–11. [Google Scholar] [CrossRef]
- Pinto, M.D.S.; Carvalho, J.E.D.; Lajolo, F.M.; Genovese, M.I.; Shetty, K. Evaluation of antiproliferative, anti-type 2 diabetes, and antihypertension potentials of ellagitannins from strawberries (Fragaria x ananassa Duch.) using in vitro models. J. Med. Food 2010, 13, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Russell, R.M.; Lischner, N.; Prior, R. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J. Nutr. 1998, 128, 2383–2390. [Google Scholar] [PubMed]
- Miyazawa, T.; Nakagawa, K.; Kudo, M.; Muraishi, K.; Someya, K. Direct intestinal absorption of red fruit anthocyanins, Cyanidin-3-glucoside and Cyanidin-3,5-diglucoside, into rats and humans. J. Agric. Food Chem. 1999, 47, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hara, H.; Chiji, H.; Kasai, K. Gastroprotective effect of red pigments in black chokeberry fruit (Aronia. melanocarpa Elliot) on acute gastric hemorrhagic lesions in rats. J. Agric. Food Chem. 2004, 52, 2226–2229. [Google Scholar] [CrossRef] [PubMed]
- Hwan, P.B.; Khanal, T.; Choi, J.M.; Chung, Y.C.; Jeong, H.G. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Jiao, H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Yu, Y.M.; Chiang, S.Y.; Tseng, C.Y. Ellagic acid suppresses oxidised low-density lipoprotein-induced aortic smooth muscle cell proliferation: Studies on the activation of extracellular signal-regulated kinase 1/2 and proliferating cell nuclear antigen expression. Br. J. Nutr. 2008, 99, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M.; Chang, W.C.; Wu, C.H.; Chiang, S.Y. Reduction of oxidative stress and apoptosis in hyperlipidemic rabbits by ellagic acid. J. Nutr. Biochem. 2005, 16, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.D.S.; Kwon, Y.I.; Apostolidis, E.; Lajolo, F.M.; Genovese, M.I.; Shetty, K. Functionality of bioactive compounds in Brazilian strawberry (Fragariax ananassa Duch.) cultivars: Evaluation of hyperglycemia and hypertension potential using in vitro models. J. Agric. Food Chem. 2008, 56, 4386–4392. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.J.; Hager, A.; Howard, L.R. Whole berries versus berry anthocyanins: Interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J. Agric. Food Chem. 2008, 56, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Abdulazeez, S.S. Effects of freeze-dried Fragaria x ananassa powder on alloxan-induced diabetic complications in Wistar rats. J. Taibah Univ. Med. Sci. 2014, 9, 268–273. [Google Scholar] [CrossRef]
- Abdulazeez, S.S. Freeze dried strawberry powder ameliorates alloxan induced hyperlipidemia in diabetic rats. Biomed. Res. 2015, 26, 77–81. [Google Scholar]
- Chebil, L.; Humeau, C.; Anthoni, J.; Dehez, F.; Engasser, J.; Ghoul, M. Solubility of flavonoids in organic solvents. J. Chem. Eng. Data 2007, 52, 1552–1556. [Google Scholar] [CrossRef]
- Ferreira, O.; Pinho, S.P. Solubility of flavonoids in pure solvents. Ind. Eng. Chem. Res. 2012, 51, 6586–6590. [Google Scholar] [CrossRef]
- Mandave, P.; Rani, S.; Kuvalekar, A.; Ranjekar, P. Antiglycation, antioxidant and antidiabetic activity of mature strawberry (Fragariax ananassa Duch.) fruits. Int. J. Appl. Biol. Pharm. 2013, 4, 168–177. [Google Scholar]
- Mandave, P.C.; Pawar, P.K.; Ranjekar, P.K.; Mantri, N.; Kuvalekar, A.A. Comprehensive evaluation of in vitro antioxidant activity, total phenols and chemical profiles of two commercially important strawberry varieties. Sci. Hort. 2014, 172, 124–134. [Google Scholar] [CrossRef]
- Rani, S.; Mandave, P.; Kuvalekar, A.; Ranjekar, P. Antiglycation, antioxidant and antidiabetic activity of strawberry (Fragariax ananassa Duch.) fruits during ripening stages. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 193–203. [Google Scholar]
- Kumawat, M.; Sharma, T.K.; Singh, I.; Singh, N.; Ghalaut, V.S.; Vardey, S.K.; Shankar, V. Antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus patients with and without nephropathy. N. Am. J. Med. Sci. 2013, 5, 213–219. [Google Scholar] [PubMed]
- Naderi, R.; Mohaddes, G.; Mohammadi, M.; Ghaznavi, R.; Ghyasi, R.; Vatankhah, A.M. Voluntary exercise protects heart from oxidative stress in diabetic rats. Adv. Pharm. Bull. 2015, 5, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kochar, N.I.; Umathe, S.N. Beneficial effects of l-arginine against diabetes-induced oxidative stress in gastrointestinal tissues in rats. Pharmacol. Rep. 2009, 61, 665–672. [Google Scholar] [CrossRef]
- Zelen, I.; Djurdjevic, P.; Popovic, S.; Stojanovic, M.; Jakovljevic, V.; Radivojevic, S.; Baskic, D.; Arsenijevic, N. Antioxidant enzymes activities and plasma levels of oxidative stress markers in B-chronic lymphocytic leukemia patients. J. BUON 2010, 15, 330–336. [Google Scholar] [PubMed]
- Mandave, P.M.; Khadke, S.; Karandikar, M.; Pandit, V.; Ranjekar, P.; Kuvalekar, A.; Mantri, N.; Interactive Research School for Health Affairs, Bharati Vidyapeeth University, Katraj, Pune, Maharashtra, India. Hematoxylin- and eosin-stained cross sections of paraffin-embedded brain and liver tissues of rats from control and experimental groups. Unpublished work. 2017. [Google Scholar]
- Basu, A.; Wilkinson, M.; Penugonda, K.; Simmons, B.; Betts, N.M.; Lyons, T.J. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: Baseline and post intervention effects. Nutr. J. 2009, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Pieszka, M.; Tombarkiewicz, B.; Roman, A.; Migdał, W.; Niedziolka, J. Effect of bioactive substances found in rapeseed, raspberry and strawberry seed oils on blood lipid profile and selected parameters of oxidative status in rats. Environ. Toxicol. Pharmacol. 2013, 36, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Jaroslawska, J.; Juskiewicz, J.; Wroblewska, M.; Jurgonski, A.; Krol, B.; Zdunczyk, Z. Polyphenol-rich strawberry pomace reduces serum and liver lipids and alters gastrointestinal metabolite formation in fructose-fed rats. J. Nutr. 2011, 141, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Fadri, R.A.; Rilma, N.; Fadil, O.; Kesuma, S. The mixture of yogurt and strawberry juice to repair blood lipid profile. Int. J. Adv. Sci. Eng. Inf. Technol. 2014, 4, 137–139. [Google Scholar]
- Basu, A.; Michael, R.; Timothy, J.L. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.C.; Joshipura, K.J.; Jiang, R.; Hu, F.B.; Hunter, D.; Smith-Warner, S.A.; Colditz, G.A.; Rosner, B.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst. 2004, 96, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Riitta, F.; Jukka, M.; Paula, H.; Georg, A. Bioavailability of quercetin from berries and the diet. Nutr. Cancer 2006, 54, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Maria, R.W.; Maria, E.P.; Daniel, R.G. Ellagic acid content in strawberries of different cultivars and ripening stages. J. Sci. Food Agric. 2003, 83, 842–845. [Google Scholar]
- Pinto, M.D.S.; Franco, M.L.; Genovese, M.I. Bioactive compounds and quantification of total ellagic acid in strawberries (Fragaria x ananassa Duch.). Food Chem. 2008, 107, 1629–1635. [Google Scholar] [CrossRef]
- Maher, P.; Dargusch, R.; Ehren, J.L.; Okada, S.; Sharma, K.; Schubert, D. Fisetin lowers methylglyoxal dependent protein glycation and limits the complications of diabetes. PLoS ONE 2011, 6, e21226. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.; Wahli, W. Peroxisome proliferator-activated receptors—A link between endocrinology and nutrition? Trends Endocrinol. Metab. 1993, 4, 291–296. [Google Scholar] [CrossRef]
- Schoonjans, K.; Staels, B.; Auwerx, J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim. Biophys. Acta 1996, 1302, 93–109. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. Eur. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Shimomura, I.; Bashmakov, Y.; Horton, J.D. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J. Biol. Chem. 1999, 274, 30028–30032. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.A.; Liang, G.; Xie, X.; Frank-Kamenetsky, M.; Fitzgerald, K.; Koteliansky, V.; Brown, M.S.; Goldstein, J.L.; Horton, J.D. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012, 15, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Konstantopoulos, N.; Lee, J.; Hansen, L.; Li, Z.W.; Karin, M.; Shoelson, S.E. Reversal of obesity and diet induced insulin resistance with salicylates or targeted disruption of IKK-β. Science 2001, 293, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. IKK-βlinks inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κβ. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Houten, S.M.; Wanders, R.J.A. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Fahey, T.; Baldwin, K. Exercise Physiology: Human Bioenergetics and Its Applications, 4th ed.; McGraw-Hill Education: Boston, MA, USA, 2004. [Google Scholar]
- Furuta, E.; Pai, S.K.; Zhan, R.; Bandyopadhyay, S.; Watabe, M.; Mo, Y.Y.; Hirota, S.; Hosobe, S.; Tsukada, T.; Miura, K.; et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008, 68, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Singh, S.B.; Wang, J.; Chung, C.C.; Salituro, G.; Karanam, B.V.; Lee, S.H.; Powles, M.; Ellsworth, K.P.; Lassman, M.E.; et al. Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 5378–5383. [Google Scholar] [CrossRef] [PubMed]
- Radenne, A.; Akpa, M.; Martel, C.; Sawadogo, S.; Mauvoisin, D.; Mounier, C. Hepatic regulation of fatty acid synthase by insulin and T3: Evidence for T3 genomic and nongenomic actions. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Montoudis, A.; Seidman, E.; Boudreau, F.; Beaulieu, J.F.; Menard, D.; Elchebly, M.; Mailhot, G.; Sane, A.T.; Lambert, M.; Delvin, E.; et al. Intestinal fatty acid binding protein regulates mitochondrion β-oxidation and cholesterol uptake. J. Lipid Res. 2008, 49, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Smathers, R.L.; Petersen, D.R. The human fatty acid-binding protein family: Evolutionary divergences and functions. Hum. Genom. 2011, 5, 170–191. [Google Scholar] [CrossRef]
- Hisanaga, Y.; Ago, H.; Nakagawa, N.; Hamada, K.; Ida, K.; Yamamoto, M.; Hori, T.; Arii, Y.; Sugahara, M.; Kuramitsu, S.; et al. Structural basis of the substrate-specific two-step catalysis of long chain fatty acyl-CoA synthetase dimer. J. Biol. Chem. 2004, 279, 31717–31726. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Black, S.M. Carnitine homeostasis, mitochondrial function, and cardiovascular disease. Drug Discov. Today Dis. Mech. 2009, 6, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Soued, M.; Staprans, I.; Gavin, L.A.; Donahue, M.E.; Huang, B.J.; Moser, A.H.; Gulli, R.; Grunfeld, C. Effect of tumor necrosis factor (TNF) on lipid metabolism in the diabetic rat. Evidence that inhibition of adipose tissue lipoprotein lipase activity is not required for TNF-induced hyperlipidemia. J. Clin. Investig. 1989, 83, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Fon, T.K.; Kuzman, D.; Seliskar, M.; Pompon, D.; Rozman, D. TNF-α interferes with lipid homeostasis and activates acute and proatherogenic processes. Physiol. Genom. 2007, 31, 216–227. [Google Scholar]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Murray, A.J.; Menassa, D.; Ashmore, T.; Nicholls, A.W.; Griffin, J.L. The contrasting roles of PPAR-δ and PPAR-γ in regulating the metabolic switch between oxidation and storage of fats in white adipose tissue. Genome Biol. 2011, 12, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickso, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Warnick, G.R.; Knopp, R.H.; Fitzpatrick, V.; Branson, L. Estimating low-density lipoprotein cholesterol by the friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin. Chem. 1990, 36, 15–19. [Google Scholar] [PubMed]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation methods. Enzymology 1978, 52, 302–310. [Google Scholar]
- Goth, L.; Nemeth, H.; Meszaros, I. Serum catalase activity for detection of hemolytic diseases. Clin. Chem. 1983, 29, 741–743. [Google Scholar] [PubMed]
- Campbell, J.E.; Oh, T.; Hurtgen, B.J.; Niemeyer, D.M.; Sheppard, F.R.; Lucca, J.J.D. Blast overpressure induced pulmonary and intestinal damage is ameliorated by post-injury decay accelerating factor injection. J. Clin. Immunol. Immunother. 2015, 2, 1–6. [Google Scholar]










| Groups | 30th Day | 36th Day | 43rd Day | 50th Day |
|---|---|---|---|---|
| Healthy Control | 111.47 ± 7.02 ** | 129.38 ± 3.02 ** | 117.48 ± 11.62 ** | 86.08 ± 7.70 ** |
| NIC-STZ Control | 353.60 ± 15.87 | 486.46 ± 45.69 | 664.19 ± 21.48 | 708.47 ± 12.66 |
| MET-treated Control | 578.98 ± 28.68 ** | 551.57 ± 6.39 ns | 555.92 ± 6.99 ** | 411.69 ± 11.02 ** |
| SWE | 531.01 ± 32.35 ** | 473.17 ± 31.53 ns | 504.18 ± 30.61 ** | 371.16 ± 15.41 ** |
| SHAE | 537.04 ± 1.1.6 ** | 589.57 ± 12.92 * | 482.54 ± 2.86 ** | 400.00 ± 16.74 ** |
| SAE | 525.27 ± 2.45 ** | 518.12 ± 2.45 ns | 421.67 ± 3.58 ** | 375.71 ± 2.24 ** |
| Group | Liver Function Tests | Lipid Profile | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SGOT (U/mL) | SGPT (U/mL) | ALP (U/mL) | BIL (mg/dL) | TC (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | VLDL (mg/dL) | TG (mg/dL) | |
| Healthy Control | 38.43 ± 4.03 ** | 17.32 ± 1.16 ** | 23.39 ± 0.56 ** | 0.58 ± 0.05 ** | 62.24 ± 2.37 ** | 28.73 ± 3.25 ** | 27.4 ± 2.29 ** | 6.13 ± 0.46 ** | 30.62 ± 2.30 ** |
| NIC-STZ Control | 346.94 ± 5.97 | 83.54 ± 2.20 | 134.91 ± 2.02 | 1.78 ± 0.05 | 213.69 ± 2.49 | 55.99 ± 6.33 | 138.65 ± 7.70 | 19.01 ± 0.34 | 95.12 ± 1.69 |
| MET-treated Control | 261.48 ± 6.56 ** | 82.76 ± 2.82 ns | 127.8 ± 4.32 ns | 2.51 ± 0.11 ns | 174.58 ± 3.29 ** | 23.59 ± 3.91 ** | 137.82 ± 5.37 ns | 13.19 ± 0.54 ** | 65.96 ± 2.70 ** |
| SWE | 104.54 ± 7.98 ** | 25.83 ± 1.38 ** | 119.66 ± 4.29 ** | 1.14 ± 0.08 ns | 125.17 ± 4.45 ** | 32.06 ± 3.02 ** | 81.77 ± 5.29 ** | 11.34 ± 1.72 ** | 56.71 ± 8.57 ** |
| SHAE | 181.84 ± 4.39 ** | 53.7 ± 5.90 ** | 109.4 ± 2.23 ** | 2.44 ± 0.09 ns | 98.59 ± 2.93 ** | 41.5 ± 4.45 ns | 45.53 ± 5.57 ** | 11.56 ± 1.58 ** | 57.86 ± 7.88 ** |
| SAE | 347.73 ± 0.97 ns | 49 ± 4.41 ** | 146.8 ± 0.03 * | 3.96 ± 0.50 ** | 127.13 ± 0.45 ** | 58.23 ± 1.76 ns | 51.57 ± 1.35 ** | 17.33 ± 0.08 ns | 86.6 ± 0.39 ns |
| Group | % mg |
|---|---|
| Healthy Control | 1.38 ± 0.11 ** |
| NIC-STZ Control | 3.03 ± 0.10 |
| MET-treated Control | 1.86 ± 0.20 ** |
| SWE | 1.73 ± 0.07 ** |
| SHAE | 1.74 ± 0.07 ** |
| SAE | 2.97 ± 0.01 ns |
| Groups | MDA (μM/g Protein) | CAT (kU/L) × 102 |
|---|---|---|
| Healthy Control | 3.147 ± 0.06 ** | 24.05 ± 0.37 * |
| NIC-STZ Control | 8.628 ± 1.54 ##,++ | 19.09 ± 0.16 + |
| MET-treated Control | 4.622 ± 0.30 ** | 24.76 ± 2.36 * |
| SWE | 5.95 ± 0.95 ** | 27.66 ± 1.10 ** |
| SHAE | 3.59 ± 0.62 ** | 22.05 ± 1.77 |
| SAE | 7.25 ± 0.03 ## | 37.70 ± 0.12 ++,** |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandave, P.; Khadke, S.; Karandikar, M.; Pandit, V.; Ranjekar, P.; Kuvalekar, A.; Mantri, N. Antidiabetic, Lipid Normalizing, and Nephroprotective Actions of the Strawberry: A Potent Supplementary Fruit. Int. J. Mol. Sci. 2017, 18, 124. https://doi.org/10.3390/ijms18010124
Mandave P, Khadke S, Karandikar M, Pandit V, Ranjekar P, Kuvalekar A, Mantri N. Antidiabetic, Lipid Normalizing, and Nephroprotective Actions of the Strawberry: A Potent Supplementary Fruit. International Journal of Molecular Sciences. 2017; 18(1):124. https://doi.org/10.3390/ijms18010124
Chicago/Turabian StyleMandave, Pallavi, Suresh Khadke, Manjiri Karandikar, Vijaya Pandit, Prabhakar Ranjekar, Aniket Kuvalekar, and Nitin Mantri. 2017. "Antidiabetic, Lipid Normalizing, and Nephroprotective Actions of the Strawberry: A Potent Supplementary Fruit" International Journal of Molecular Sciences 18, no. 1: 124. https://doi.org/10.3390/ijms18010124
APA StyleMandave, P., Khadke, S., Karandikar, M., Pandit, V., Ranjekar, P., Kuvalekar, A., & Mantri, N. (2017). Antidiabetic, Lipid Normalizing, and Nephroprotective Actions of the Strawberry: A Potent Supplementary Fruit. International Journal of Molecular Sciences, 18(1), 124. https://doi.org/10.3390/ijms18010124