Aquaporin-Mediated Water and Hydrogen Peroxide Transport Is Involved in Normal Human Spermatozoa Functioning
Abstract
:1. Introduction
2. Results
2.1. Semen Characteristics in Normospermic and Sub-Fertile Subjects
2.2. Immunoblotting of AQP3, -7, -8 and -11 Protein Expression in Human Ejaculated Semen from Normospermic Subjects
2.3. Aquaporins Immunolocalization in Human Spermatozoa from Normospermic Subjects
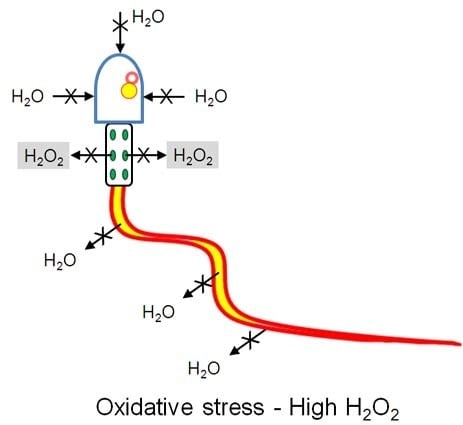
2.4. Effect of Oxidative Stress on Water Permeability of Human Ejaculated Semen from Normospermic and Sub-Fertile Subjects
2.5. Temperature Dependence of the Osmotic Water Permeability of Human Ejaculated Semen from Normospermic Subjects
2.6. Reversible Effect of the H2O2 and HgCl2 Treatment on the Osmotic Water Permeability of Human Ejaculated Semen from Normospermic Subjects
2.7. Effect of Mercury Chloride (HgCl2) Treatment on Hydrogen Peroxide Permeability and Motility of Human Ejaculated Semen from Normospermic and Sub-Fertile Subjects
3. Discussion
4. Materials and Methods
4.1. Sperm Samples
4.2. Routine Sperm Analysis
4.2.1. Macroscopic Analysis
4.2.2. Determination of Sperm Count and Motility
4.2.3. Determination of Sperm Morphology
4.2.4. Determination of Sperm Viability
4.3. Immunoblotting
4.4. Immunocytochemistry
4.5. Water Permeability Measurements
4.6. Hydrogen Peroxide Permeability Measurements
4.7. Protein Content
4.8. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species |
| CM-H2DCFDA | 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester |
| SOD | superoxide dismutase |
| AQP | aquaporins |
| FACS | fluorescence-activated cell sorter |
| WHO | World Health Organization |
| PR | progressive |
| NP | non-progressive |
| DTT | dithiothreitol |
References
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World. J. Mens Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lanzafame, F.M.; La Vignera, S.; Vicari, E.; Calogero, A.E. Oxidative stress and medical antioxidant treatment in male infertility. Reprod. Biomed. Online 2009, 19, 638–659. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J. Androl. 1992, 13, 368–378. [Google Scholar] [PubMed]
- De Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J. Androl. 1992, 13, 379–386. [Google Scholar] [PubMed]
- De Lamirande, E.; Eiley, D.; Gagnon, C. Inverse relationship between the induction of human sperm capacitation and spontaneous acrosome reaction by various biological fluids and the superoxide scavenging capacity of these fluids. Int. J. Androl. 1993, 16, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Griveau, J.F.; Renard, P.; Le Lannou, D. An in vitro promoting role for hydrogen peroxide in human sperm capacitation. Int. J. Androl. 1994, 17, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Kuribayashi, Y.; Gagnon, C. Effect of sperm lipid peroxidation on fertilization. J. Androl. 1996, 17, 151–157. [Google Scholar] [PubMed]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2011. [Google Scholar] [CrossRef] [PubMed]
- Sikka, S.C. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front. Biosci. 1996, 1, e78–e86. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Curson, B. Toxic effect and action of dead sperm on diluted bovine semen. J. Dairy Sci. 1972, 55, 614–620. [Google Scholar] [CrossRef]
- Kovalski, N.N.; de Lamirande, E.; Gagnon, C. Reactive oxygen species generated by human neutrophils inhibit sperm motility: Protective effect of seminal plasma and scavengers. Fertil. Steril. 1992, 58, 809–816. [Google Scholar] [CrossRef]
- Aitken, R.J.; West, K.; Buckingham, D. Leukocytic infiltration into the human ejaculate and its association with semen quality, oxidative stress, and sperm function. J. Androl. 1994, 15, 343–352. [Google Scholar] [PubMed]
- Aitken, R.J.; Fisher, H.M.; Fulton, N.; Gomez, E.; Knox, W.; Lewis, B.; Irvine, S. Reactive oxygen species generation by human spermatozoa is induced by exogenous NADPH and inhibited by the flavoprotein inhibitors diphenylene iodonium and quinacrine. Mol. Reprod. Dev. 1997, 47, 468–482. [Google Scholar] [CrossRef]
- Hendin, B.N.; Kolettis, P.N.; Sharma, R.K.; Thomas, A.J.; Agarwal, A. Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J. Urol. 1999, 161, 1831–1834. [Google Scholar] [CrossRef]
- De Lamirande, E.; Gagnon, C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic. Biol. Med. 1993, 14, 157–166. [Google Scholar] [CrossRef]
- Gil-Guzman, E.; Ollero, M.; Lopez, M.C.; Sharma, R.K.; Alvarez, J.G.; Thomas, A.J.; Agarwal, A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum. Reprod. 2001, 16, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Sabeur, K.; Ball, B.A. Detection of superoxide anion generation by equine spermatozoa. Am. J. Vet. Res. 2006, 67, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Sabeur, K.; Ball, B.A. Characterization of NADPH oxidase 5 in equine testis and spermatozoa. Reproduction 2007, 134, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Cadenas, E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000, 475, 121–126. [Google Scholar] [CrossRef]
- Laforenza, U.; Bottino, C.; Gastaldi, G. Mammalian aquaglyceroporin function in metabolism. Biochim. Biophys. Acta 2016, 1858, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Medraño-Fernandez, I.; Bestetti, S.; Bertolotti, M.; Bienert, G.P.; Bottino, C.; Laforenza, U.; Rubartelli, A.; Sitia, R. Stress regulates aquaporin-8 permeability to impact cell growth and survival. Antioxid. Redox Signal. 2016, 24, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016, 471, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Peng, H.; Lei, L.; Zhang, Y.; Kuang, H.; Cao, Y.; Shi, Q.X.; Ma, T.; Duan, E. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011, 21, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Kageyama, Y.; Okada, Y.; Kawakami, S.; Kihara, K.; Ishibashi, K.; Sasaki, S. Localization of aquaporin-7 in human testis and ejaculated sperm: Possible involvement in maintenance of sperm quality. J. Urol. 2004, 172, 2073–2076. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H. Aquaporins in spermatozoa and testicular germ cells: Identification and potential role. Asian J. Androl. 2010, 12, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Callies, C.; Tüttelmann, F.; Kliesch, S.; Cooper, T.G. Aquaporins in the human testis and spermatozoa—Identification, involvement in sperm volume regulation and clinical relevance. Int. J. Androl. 2010, 33, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Cooper, T.G. Aquaporin AQP11 in the testis: Molecular identity and association with the processing of residual cytoplasm of elongated spermatids. Reproduction 2010, 139, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Chauvigné, F.; Boj, M.; Finn, R.N.; Cerdà, J. Mitochondrial aquaporin-8-mediated hydrogen peroxide transport is essential for teleost spermatozoon motility. Sci. Rep. 2015, 5, 7789. [Google Scholar] [CrossRef] [PubMed]
- Durairajanayagam, D.; Agarwal, A.; Ong, C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod. Biomed. Online 2015, 30, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Bahat, A.; Eisenbach, M. Sperm thermotaxis. Mol. Cell. Endocrinol. 2006, 252, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Bahat, A.; Caplan, S.R.; Eisenbach, M. Thermotaxis of human sperm cells in extraordinarily shallow temperature gradients over a wide range. PLoS ONE 2012, 7, e41915. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Song, Y.; Zhao, D.; Verkman, A.S. Phenotype analysis of aquaporin-8 null mice. Am. J. Physiol. Cell Physiol. 2005, 288, C1161–C1170. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F.; He, R.H.; Sun, C.C.; Zhang, Y.; Meng, Q.X.; Ma, Y.Y. Function of aquaporins in female and male reproductive systems. Hum. Reprod. Update 2006, 12, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tan, Y.J.; Qu, F.; Sheng, J.Z.; Huang, H.F. Functions of water channels in male and female reproductive systems. Mol. Asp. Med. 2012, 33, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Shannonhouse, J.L.; Urbanski, H.F.; Woo, S.L.; Fong, L.A.; Goddard, S.D.; Lucas, W.F.; Jones, E.R.; Wu, C.; Morgan, C. Aquaporin-11 control of testicular fertility markers in Syrian hamsters. Mol. Cell. Endocrinol. 2014, 391, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Mazzone, A.; Cho, Y.S.; Valenti, G.; Svelto, M. Expression and localization of the aquaporin-8 water channel in rat testis. Biol. Reprod. 2001, 64, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Mazzone, A.; Bizzoca, A.; Svelto, M. Possible involvement of aquaporin-7 and -8 in rat testis development and spermatogenesis. Biochem. Biophys. Res. Commun. 2001, 288, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Callies, C.; Rojek, A.; Nielsen, S.; Cooper, T.G. Aquaporin isoforms involved in physiological volume regulation of murine spermatozoa. Biol. Reprod. 2009, 80, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Sohara, E.; Ueda, O.; Tachibe, T.; Hani, T.; Jishage, K.; Rai, T.; Sasaki, S.; Uchida, S. Morphologic and functional analysis of sperm and testes in Aquaporin 7 knockout mice. Fertil. Steril. 2007, 87, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Cooper, T.G. Effects of the ion-channel blocker quinine on human sperm volume, kinematics and mucus penetration, and the involvement of potassium channels. Mol. Hum. Reprod. 2001, 7, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Verbavatz, J.M.; Valenti, G.; Lui, B.; Sabolić, I. Localization of the CHIP28 water channel in reabsorptive segments of the rat male reproductive tract. Eur. J. Cell Biol. 1993, 61, 264–273. [Google Scholar] [PubMed]
- Boj, M.; Chauvigné, F.; Cerdà, J. Aquaporin biology of spermatogenesis and sperm physiology in mammals and teleosts. Biol. Bull. 2015, 229, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, C.E.; Mazur, P.; Peter, A.T.; Critser, J.K. Osmotic tolerance limits and properties of murine spermatozoa. Biol. Reprod. 1996, 55, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, N.; Mayorga, L.S. Acrosomal swelling and membrane docking are required for hybrid vesicle formation during the human sperm acrosome reaction. Biol. Reprod. 2009, 81, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.; di Virgilio, F.; Foresta, C. Involvement of osmo-sensitive calcium influx in human sperm activation. Mol. Hum. Reprod. 1996, 2, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, M.; Bestetti, S.; García-Manteiga, J.M.; Medraño-Fernandez, I.; dal Mas, A.; Malosio, M.L.; Sitia, R. Tyrosine kinase signal modulation: a matter of H2O2 membrane permeability? Antioxid. Redox Signal. 2013, 19, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Almasalmeh, A.; Krenc, D.; Wu, B.; Beitz, E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J. 2014, 281, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Atochina-Vasserman, E.N.; Biktasova, A.; Abramova, E.; Cheng, D.S.; Polosukhin, V.V.; Tanjore, H.; Takahashi, S.; Sonoda, H.; Foye, L.; Venkov, C.; et al. Aquaporin 11 insufficiency modulates kidney susceptibility to oxidative stress. Am. J. Physiol. Ren. Physiol. 2013, 304, F1295–F1307. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gao, D.; Preston, G.M.; McGann, L.E.; Benson, C.T.; Critser, E.S.; Critser, J.K. High water permeability of human spermatozoa is mercury-resistant and not mediated by CHIP28. Biol. Reprod. 1995, 52, 913–919. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Laemmli, U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS ONE 2013, 8, e54474. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Gastaldi, G.; Grazioli, M.; Cova, E.; Tritto, S.; Faelli, A.; Calamita, G.; Ventura, U. Expression and immunolocalization of aquaporin-7 in rat gastrointestinal tract. Biol. Cell 2005, 97, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U. Water channel proteins in the gastrointestinal tract. Mol. Asp. Med. 2012, 33, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Cova, E.; Gastaldi, G.; Tritto, S.; Grazioli, M.; LaRusso, N.F.; Splinter, P.L.; D‘Adamo, P.; Tosco, M.; Ventura, U. Aquaporin-8 is involved in water transport in isolated superficial colonocytes from rat proximal colon. J. Nutr. 2005, 135, 2329–2336. [Google Scholar] [PubMed]
- Yeung, Y.G.; Stanley, E.R. A solution for stripping antibodies from polyvinylidene fluoride immunoblots for multiple reprobing. Anal. Biochem. 2009, 389, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Miceli, E.; Gastaldi, G.; Scaffino, M.; Ventura, U.; Fontana, J.; Orsenigo, M.; Corazza, G. Solute transporters and aquaporins are impaired in celiac disease. Biol. Cell 2010, 102, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A. Water permeability measurement in living cells and complex tissues. J. Membr. Biol. 2000, 173, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Tritto, S.; Gastaldi, G.; Zelenin, S.; Grazioli, M.; Orsenigo, M.; Ventura, U.; Laforenza, U.; Zelenina, M. Osmotic water permeability of rat intestinal brush border membrane vesicles: involvement of aquaporin-7 and aquaporin-8 and effect of metal ions. Biochem. Cell Biol. 2007, 85, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]








| Semen Parameters | Normospermic (n = 53) | Sub-Fertile (n = 26) |
|---|---|---|
| Semen volume (mL) | 3.55 ± 0.20 | 4.22 ± 0.34 |
| Sperm concentration (106/mL) | 69.81 * ± 5.58 | 17.50 ± 4.29 |
| Progressive motility (PR; %) | 51.70 * ± 1.76 | 21.81 ± 2.90 |
| Motile sperm count (106/mL) | 37.02 * ± 3.62 | 3.89 ± 1.05 |
| Non-progressive motility (NP; %) | 9.7 ± 0.82 | 9.15 ± 1.14 |
| Total motility (PR + NP; %) | 61.40 * ± 1.78 | 30.96 ± 3.11 |
| Morphology (% normal) | 2.16 * ± 0.26 | 0.80 ± 0.18 |
| Semen Parameters | Normospermic (n = 12) | Sub-Fertile (n = 12) | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Progressive motility (PR; %) | 50.17 * ± 3.37 | 39.92 ± 4.99 | 22.17 ± 3.18 | 19.42 ± 3.53 |
| Motile sperm count | 45.76 * ± 10.74 | 35.10 ± 10.12 | 5.71 ± 1.66 | 4.76 ± 1.43 |
| Non-progressive motility (NP; %) | 6.50 ± 0.91 | 6.17 ± 0.97 | 7.08 * ± 1.17 | 3.33 ± 0.40 |
| Total motility (PR + NP; %) | 56.67 * ± 3.61 | 46.08 ± 4.89 | 29.25 * ± 3.29 | 22.75 ± 3.4 |
| Vitality (%) | 77.25 * ± 3.83 | 65.50 ± 5.07 | 73.5 * ± 2.07 | 66.42 ± 2.52 |
| Semen Parameters | Normospermic (n = 8) | Sub-Fertile (n = 4) | ||
|---|---|---|---|---|
| Ctr | HgCl2 | Ctr | HgCl2 | |
| Progressive motility (%) | 52.5 ± 4.0 | 43.1 * ± 4.5 | 32.8 ± 3.7 | 31.3 ± 3.4 |
| Non-progressive motility (%) | 8.1 ± 1.5 | 6.4 ± 1.0 | 4.3 ± 1.1 | 4.0 ± 0.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laforenza, U.; Pellavio, G.; Marchetti, A.L.; Omes, C.; Todaro, F.; Gastaldi, G. Aquaporin-Mediated Water and Hydrogen Peroxide Transport Is Involved in Normal Human Spermatozoa Functioning. Int. J. Mol. Sci. 2017, 18, 66. https://doi.org/10.3390/ijms18010066
Laforenza U, Pellavio G, Marchetti AL, Omes C, Todaro F, Gastaldi G. Aquaporin-Mediated Water and Hydrogen Peroxide Transport Is Involved in Normal Human Spermatozoa Functioning. International Journal of Molecular Sciences. 2017; 18(1):66. https://doi.org/10.3390/ijms18010066
Chicago/Turabian StyleLaforenza, Umberto, Giorgia Pellavio, Anna Lisa Marchetti, Claudia Omes, Federica Todaro, and Giulia Gastaldi. 2017. "Aquaporin-Mediated Water and Hydrogen Peroxide Transport Is Involved in Normal Human Spermatozoa Functioning" International Journal of Molecular Sciences 18, no. 1: 66. https://doi.org/10.3390/ijms18010066
APA StyleLaforenza, U., Pellavio, G., Marchetti, A. L., Omes, C., Todaro, F., & Gastaldi, G. (2017). Aquaporin-Mediated Water and Hydrogen Peroxide Transport Is Involved in Normal Human Spermatozoa Functioning. International Journal of Molecular Sciences, 18(1), 66. https://doi.org/10.3390/ijms18010066