Absorption and Emission Spectroscopic Investigation of Thermal Dynamics and Photo-Dynamics of the Rhodopsin Domain of the Rhodopsin-Guanylyl Cyclase from the Nematophagous Fungus Catenaria anguillulae
Abstract
:1. Introduction
2. Results
2.1. Absorption and Emission Behavior of Freshly Thawed CaRh
2.1.1. Absorption Behavior of CaRh
2.1.2. Fluorescence Behavior of CaRh
2.2. Thermal CaRh Behavior
2.2.1. Apparent CaRh Melting Temperature
2.2.2. Temporal Absorption Development of CaRh at 3.5 °C
2.2.3. Temporal Absorption Development of CaRh at 20.5 °C
2.3. Photo-Excitation Dynamics of CaRh
2.3.1. Photocycle Dynamics of CaRh
2.3.2. Photo-Degradation Dynamics of CaRh
3. Discussion
3.1. Behavior of CaRh from Catenaria anguillulae
3.1.1. Spectral and Thermal Studies
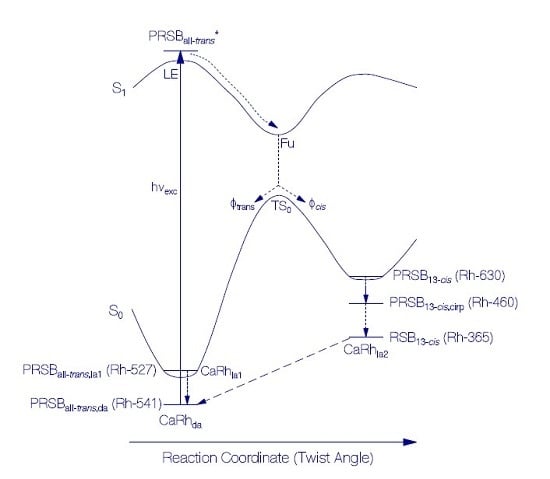
3.1.2. Photocycle Studies
Primary All-trans—13-cis Photocycle of Initially Dark-Adapted CaRh
Photo-Induced all-trans Back-Isomerization and Opsin Restructuring Cycle
Secondary Photocycle of Light-Adapted CaRhla1
Photocycle Parameters
3.2. Comparision of Behavior of CaRh from Catenaria anguillulae with Behavior of BeRh from Blastocladiella emersonii
3.3. Comparision of Photocycle Behavior of CaRh from Catenaria anguillulae with Photocycle Behavior of Channelrhodopsin ChR2 from Chlamydomonas reinhardtii
4. Materials and Methods
4.1. Sample Preparation
4.2. Spectroscopic Investigations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BeRh | Rhodopsin domain of the rhodopsin-guanylyl cyclase from Blastocladiella emersonii |
| BeRhGC | Rhodopsin-guanylyl cyclase from Blastocladiella emersonii |
| cAMP | Cyclic adenosine monophosphate |
| CaRh | Rhodopsin domain of the rhodopsin-guanylyl cyclase from Catenaria anguillulae |
| CaRhGC | Rodopsin-guanylyl cyclase from Catenaria anguillulae |
| ChR2 | Channelrhodopsin-2 |
| cGMP | Cyclic guanosine monophosphate |
| CHS | Cholesteryl hemisuccinate |
| DDM | n-dodecyl β-d-maltoside |
| FWHM | Full width at half maximum |
| Fu | Funnel state |
| G | Ground-state |
| GTP | Guanosine triphosphate |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid |
| I | Intermediate state at TS0 transition state position |
| IC | Internal conversion |
| K | K-state in photocycle (PRSB13-cis) |
| L | L-state in photocycle (PRSB13-cis,cirp) |
| LE | Locally excited state |
| LED | Light emitting diode |
| M | M-state in photocycle (RSB13-cis) |
| MOPS | 3-(N-morpholino) propanesulfonic acid |
| Prod | Photoproduct |
| PRSB | Protonated rerinal Schiff base |
| Retxxx | Retinal photoproduct with absorption maximum at wavelength xxx nm |
| Rh-xxx | Rhodopsin isomer with absorption maximum at wavelength xxx nm |
| RSB | Retinal Schiff base |
| Trp | Tryptophan |
| TS0 | Transition state on S0 ground-state potential energy surface |
| Tyr | Tyrosine |
Indices
| cirp | Counter ion repositioned |
| da | Dark-adapted |
| F | Fluorescence |
| la | Light-adapted |
| la1 | Light-adapted protonated retinal Schiff base ground-state |
| la2 | Light-adapted deprotonated retinal Schiff base |
| Pr | Probe |
| Prod | Photoproduct |
Symbols
| Symbol | Name | Unit |
| EF | Fluorescence quantum distribution | nm−1 |
| gLED | Normalized spectral distribution of excitation LED | |
| Iexc | Excitation light intensity | W cm−2 |
| Isat | Excitation saturation intensity | W cm−2 |
| l | Length | cm |
| lexc | Sample length in excitation direction | cm |
| n | Refractive index | |
| N | Number density | cm−3 |
| Pexc | Light excitation power | W |
| rpm | Rotations per minute | min−1 |
| t | Time | s, min, or h |
| T | Transmission | |
| tm | Protein melting time | h or d |
| texc | Time of sample excitation | s |
| tres | Time resolution | s |
| wexc | Excitation energy density | J cm−2 |
| α | Attenuation coefficient | cm−1 |
| αa | Absorption coefficient | cm−1 |
| αs | Scattering coefficient | cm−1 |
| γ | Scattering exponential factor | |
| ϑ | Temperature | °C |
| ϑm | Apparent melting temperature | °C |
| ĸla1 | Limiting fraction of excited CaRhda* converted to CaRhla1 at high excitation intensity | |
| ĸla2 | Limiting fraction of excited CaRhda* converted to CaRhla2 at high excitation intensity | |
| λ | Wavelength | nm |
| λF,exc | Fluorescence excitation wavelength | nm |
| λF,max | Wavelength position of maximum fluorescence emission | nm |
| Mean fluorescence wavelength | nm | |
| ν | Frequency | Hz |
| Wavenumber | cm−1 | |
| Fluorescence Stokes shift | cm−1 | |
| Fluorescence spectral half-width (FWHM) | cm−1 | |
| Absorption spectral half-width (FWHM) of S0–S1 transition | cm−1 | |
| σa | Absorption cross-section | cm2 |
| Absorption band cross-section strength | cm2 | |
| τF | Fluorescence lifetime | ps |
| τrad | Radiative lifetime | ns |
| τrec | Recovery time constant | s |
| Φcis | Quantum yield of trans-cis isomerization | |
| Φd | Quantum yield of photo-degradation | |
| ΦF | Fluorescence quantum yield | |
| Φtrans | Quantum yield of trans back isomerization | |
| ∆nph,abs | Increment of absorbed excitation photons | cm−2 |
| N | Increment of length-integrated number density | cm−2 |
References
- Gupta, H.C.; Singh, K.P. Effect of sodium chloride on biology of Catenaria anguillulae. Mycobiology 2002, 30, 219–224. [Google Scholar] [CrossRef]
- Singh, K.P.; Vaish, S.S.; Kumar, N.; Singh, K.D.; Kumari, M. Catenaria anguillulae as an efficient biological control agent of Anguina tritici in vitro. Biol. Control 2012, 61, 185–193. [Google Scholar] [CrossRef]
- Avelar, G.M.; Schumacher, R.I.; Zaini, P.A.; Leonard, G.; Richards, T.A.; Gomes, S.L. A rhodopsin-guanylyl cyclase gene fusion functions in visual perception in a fungus. Curr. Biol. 2014, 24, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Nagpal, J.; Schneider, M.W.; Kozjak-Pavlovic, V.; Nagel, G.; Gottschalk, A. Optogenetic manipulation of cGMP in cells and animals by the tightly light-regulated guanylyl-cyclase opsin CyclOp. Nat. Commun. 2015, 6, 8046. [Google Scholar] [CrossRef] [PubMed]
- Scheib, U.; Broser, M.; Gao, S.; Constantin, O.; Stehfest, K.; Gee, C.E.; Oertner, T.G.; Nagel, G.; Hegemann, P. Rhodopsin-cyclases for light-induced cGMP & cAMP production. 2017, in press. [Google Scholar]
- Penzkofer, A.; Scheib, U.; Hegemann, P.; Stehfest, K. Absorption and emission spectroscopic investigation of thermal dynamics and photo-dynamics of the rhodopsin domain of the rhodopsin-guanylyl cyclase from the aquatic fungus Blastocladiella emersonii. BAOJ Phys. 2016, 2, 6. [Google Scholar]
- Penzkofer, A.; Shirdel, J.; Zirak, P.; Breitkreuz, H.; Wolf, E. Protein aggregation studied by forward light scattering and light transmission analysis. Chem. Phys. 2007, 342, 55–63. [Google Scholar] [CrossRef]
- Ernst, O.P.; Lodowski, D.T.; Elstner, M.; Hegemann, P.; Brown, L.S.; Kandori, H. Microbial and animal rhodopsins: Structures, functions, and molecular mechanisms. Chem. Rev. 2014, 114, 126–163. [Google Scholar] [CrossRef] [PubMed]
- Penzkofer, A.; Luck, M.; Mathes, T.; Hegemann, P. Bistable retinal Schiff base photodynamics of histidine kinase rhodopsin HKR1 from Chlamydomonas reinhardtii. Photochem. Photobiol. 2014, 90, 773–785. [Google Scholar] [PubMed]
- Strickler, S.J.; Berg, R.A. Relationship between absorption intensity and fluorescence lifetime of molecules. J. Chem. Phys. 1962, 37, 814–822. [Google Scholar] [CrossRef]
- Birks, J.B.; Dyson, D.J. The relations between the fluorescence and absorption properties of organic molecules. Proc. Roy. Soc. London Ser. A 1963, 275, 135–148. [Google Scholar] [CrossRef]
- Deshpande, A.V.; Beidoun, A.; Penzkofer, A.; Wagenblast, G. Absorption and emission spectroscopic investigation of cyanovinyldiethylaniline dye vapors. Chem. Phys. 1990, 142, 123–131. [Google Scholar] [CrossRef]
- Lindsey, J. PhotochemCAD spectra by category. Available online: http://omlc.ogi.edu/spectra/PhotochemCAD/html/ (accessed on 30 November 2004).
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Principles of Molecular Photochemistry. An introduction; University Science Books: Sausalito, CA, USA, 2009. [Google Scholar]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural changes accompanying intramolecular electron transfer: Focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 2003, 103, 3899–4031. [Google Scholar] [CrossRef] [PubMed]
- Förster, T.H. Fluoreszenz Organischer Verbindungen; Vandenhoeck und Ruprecht: Göttingen, Germany, 1951. [Google Scholar]
- Valeur, B. Molecular Fluorescence; Principles and Applications; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Chen, R.R. Fluorescence quantum yields of tryptophan and tyrosine. Anal. Lett. 1967, 1, 35–42. [Google Scholar] [CrossRef]
- Eisinger, J.; Navon, G. Fluorescence quenching and isotope effect in tryptophan. J. Chem. Phys. 1969, 50, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.P.; Steiner, R.F. The influence of solvent and temperature upon the fluorescence of indole derivatives. J. Phys. Chem. 1970, 74, 4480–4490. [Google Scholar] [CrossRef]
- Penzkofer, A.; Stierl, M.; Hegemann, P.; Kateriya, S. Thermal protein unfolding in photo-activated adenylate cyclase nano-clusters from the amoeboflagellate Naegleria gruberi NEG-M strain. J. Photochem. Photobiol. A Chem. 2011, 225, 42–51. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Biochemistry, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 482–505. [Google Scholar]
- Becker, R.S.; Freedman, K. A comprehensive investigation of the mechanism and photophysics of isomerization of a protonated and unprotonated Schiff base of 11-cis-retinal. J. Am. Chem. Soc. 1985, 107, 1477–1485. [Google Scholar] [CrossRef]
- Weigand, R.; Rotermund, F.; Penzkofer, A. Aggregation dependent absorption reduction of indocyanine green. J. Phys. Chem. A 1997, 101, 7729–7734. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Yin, J.J.; Cherng, S.H.; Yan, J.; Mei, J.; Chen, T.; Boudreau, M.D.; Howard, P.C.; Wamer, W.G. Photodecomposition of vitamin A and photobiological implications to the skin. Photochem. Photobiol. 2007, 83, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Kateriya, S.; Nagel, G.; Bamberg, E.; Hegemann, P. “Vision” in single-celled algae. News Physiol. Sci. 2004, 19, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, P. Algal sensory photoreceptors. Annu. Rev. Plant Biol. 2008, 59, 167–189. [Google Scholar] [CrossRef] [PubMed]
- Luck, M.; Mathes, T.; Bruun, S.; Fudim, R.; Hagedorn, R.; Nguyen, T.M.T.; Kateriya, S.; Kennis, J.T.M.; Hildebrandt, P.; Hegemann, P. A photochromic histidine kinase rhodopsin (HKR1) that is bimodally switched by ultraviolet and blue light. J. Biol. Chem. 2012, 287, 40083–40090. [Google Scholar] [CrossRef] [PubMed]
- Scheib, U.; Stehfest, K.; Gee, C.E.; Körschen, H.G.; Fudim, R.; Oertner, T.G.; Hegemann, P. The rhodopsin-guanylyl cyclase of the aquatic fungus Blastocladiella emersonii enables fast optical control of cGMP signaling. Sciencesignaling 2015, 8, rs8. [Google Scholar] [CrossRef] [PubMed]
- Trieu, M.M.; Devine, E.L.; Lamarche, L.B.; Ammerman, A.E.; Greco, J.A.; Birge, R.R.; Theobald, D.L.; Oprian, D.D. Expression, purification, and spectral tuning of RhoGC, a retenylidene/guanylyl cyclase fusion protein and optogenetics tool from the aquatic fungus Blastocladiella emersonii. J. Biol. Chem. 2017, 292, 10379–10389. [Google Scholar] [CrossRef] [PubMed]
- Penzkofer, A.; Blau, W. Theoretical analysis of S1-state lifetime measurements of dyes with picoseconds laser pulses. Opt. Quant. Electron. 1983, 15, 325–347. [Google Scholar] [CrossRef]
- Penzkofer, A. Solid state lasers. Prog. Quant. Electron. 1988, 12, 91–428. [Google Scholar] [CrossRef]
- Longstaff, C.; Calhoon, R.D.; Rando, R.R. Deprotonation of the Schiff base of rhodopsin is obligate in the activation of the G protein. Proc. Natl. Acad. Sci. USA 1986, 83, 4209–4213. [Google Scholar] [CrossRef] [PubMed]
- Lanyi, J.K.; Schobert, B. Crystallographic structure of the retinal and the protein after deprotonation of the Schiff base: The switch in the bacteriorhodopsin photocycle. J. Mol. Biol. 2002, 321, 727–737. [Google Scholar] [CrossRef]
- Kubli-Garfias, C.; Salazar-Salinas, K.; Perez-Angel, E.C.; Seminario, J.M. Light activation of the isomerization and deprotonation of the protonated Schiff base retinal. J. Mol. Model. 2011, 17, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Lanyi, J.K. Bacteriorhodopsin. Annu. Rev. Physiol. 2004, 66, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Stehfest, K.; Hegemann, P. Evolution of the channelrhodopsin photocycle model. ChemPhysChem 2010, 11, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Ritter, E.; Piwowarski, P.; Hegemann, P.; Bartl, F.J. Light-dark adaptation of channelrhodopsin C128T mutant. J. Biol. Chem. 2013, 288, 10451–10458. [Google Scholar] [CrossRef] [PubMed]
- Bruun, S.; Stoeppler, D.; Keidel, A.; Kuhlmann, U.; Luck, M.; Diehl, A.; Geiger, M.A.; Woodmansee, D.; Trauner, D.; Hegemann, P.; et al. Light-dark adaptation of channelrhodopsin involves photoconversion between the all-trans and 13-cis retinal isomers. Biochemistry 2015, 54, 5389–5400. [Google Scholar] [CrossRef] [PubMed]
- De Grip, W.J. Thermal stability of rhodopsin and opsin in some novel detergents. Methods Enzymol. 1982, 81, 256–265. [Google Scholar] [PubMed]
- Holzer, W.; Pichlmaier, M.; Penzkofer, A.; Bradley, D.D.C.; Blau, W.J. Fluorescence spectroscopic behavior of neat and blended conjugated polymer thin films. Chem. Phys. 1999, 246, 445–462. [Google Scholar] [CrossRef]
- Penzkofer, A. Photoluminescence behavior of riboflavin and lumiflavin in liquid solutions and solid films. Chem. Phys. 2012, 400, 142–153. [Google Scholar] [CrossRef]
- Sens, R. Strahlungslose Desaktivierung in Xanthen-, Oxazin- und Carbazinfarbstoffen. Ph.D. Thesis, Universität Gesamthochschule Siegen, Siegen, Germany, 1984. [Google Scholar]


















| Transition | I | II | III | IV | V |
|---|---|---|---|---|---|
| PRSB | PRSB | PRSB | PRSB | Apoprotein | |
| S0–S1 | S0–S1 | S0–S2 | S0–S3 | S0–S1 | |
| λa (nm) | 540 | 450 | 410 | 370 | 280 |
| na | 1.3334 | 1.3370 | 1.3386 | 1.3414 | 1.353 |
| λF,max (nm) | ≈690 | ≈525 | ≈588 | ≈425 | ≈330 |
| (cm−1) | ≈6000 | ≈3000 | ≈4300 | ≈5000 | |
| (nm) | 706 | 541 | 530 | 488 | 343 |
| nF | 1.3308 | 1.3334 | 1.3338 | 1.3355 | 1.3438 |
| ΦF | 1.1 × 10−5 | 8.5 × 10−5 | 2.0 × 10−4 | 4.75 × 10−4 | 0.045 |
| (cm2) | 3.3 × 10−17 | 3.3 × 10−17 | ≈3.3 × 10−18 | ≈3.3 × 10−18 | 3.0 × 10−18 |
| τrad (ns) | 8.0 | 3.6 | ≈37 | ≈29 | 9.95 |
| τF (ps) | 0.088 | 0.305 | ≈7.4 | ≈13.7 | 448 |
| Parameter | Value | Comments |
|---|---|---|
| λa,max(Gda) (nm) | 541 | texc = 0, Figure 7 |
| ∆λa(Gda) (nm) | 98.7 | texc = 0, Figure 7 |
| λa,max(M) (nm) | 365 | Figure 7 and Figure 10 |
| Φcis | 0.46 ± 0.05 | Figure 8a and Figure S5, Equations (S11, S12, S13a, S13b) |
| Φtrans | 0.54 ± 0.05 | Φtrans = 1 − Φcis |
| (ps) | ≈0.088 | ≈ τF |
| (s) | <0.0125 | Figure S5 and Figure 9a |
| (s) | 0.048 ± 0.005 | Figure S5 and Figure 9a |
| (s) | 0.123 ± 0.005 | Figure S5 and Figure 9b |
| τrec,la2 (s) | 0.35 ± 0.01 | Figure S5 and Figure 9c |
| ĸla2 | ≈0.27 | 1-ĸla1, Equation (S9) |
| Isat (W cm−2) | ≈0.0129 | Figure S4a |
| Parameter | Value | Comments |
|---|---|---|
| λa,max(Gla1) (nm) | 527 | Figure 7 |
| (nm) | 14 | λa,max(Gda)–λa,max(Gla1) |
| (nm) | 13.7 | ∆λa(Gla1)–∆λa(Gda) |
| ĸla1 | ≈0.73 | Figure 7b and Equation (S9) |
| τrec,la1 (s) | 0.8 ± 0.06 | Figure S5, S6 and Figure 9a–e |
| τrec,cis-trans (s) | <<0.8 | Figures S5 and S6 |
| Isat (W cm−2) | ≈0.00595 | Figure S4b |
| Φd(wexc = 3 J cm−2) | ≈2.6×10−4 | Figure 12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penzkofer, A.; Scheib, U.; Stehfest, K.; Hegemann, P. Absorption and Emission Spectroscopic Investigation of Thermal Dynamics and Photo-Dynamics of the Rhodopsin Domain of the Rhodopsin-Guanylyl Cyclase from the Nematophagous Fungus Catenaria anguillulae. Int. J. Mol. Sci. 2017, 18, 2099. https://doi.org/10.3390/ijms18102099
Penzkofer A, Scheib U, Stehfest K, Hegemann P. Absorption and Emission Spectroscopic Investigation of Thermal Dynamics and Photo-Dynamics of the Rhodopsin Domain of the Rhodopsin-Guanylyl Cyclase from the Nematophagous Fungus Catenaria anguillulae. International Journal of Molecular Sciences. 2017; 18(10):2099. https://doi.org/10.3390/ijms18102099
Chicago/Turabian StylePenzkofer, Alfons, Ulrike Scheib, Katja Stehfest, and Peter Hegemann. 2017. "Absorption and Emission Spectroscopic Investigation of Thermal Dynamics and Photo-Dynamics of the Rhodopsin Domain of the Rhodopsin-Guanylyl Cyclase from the Nematophagous Fungus Catenaria anguillulae" International Journal of Molecular Sciences 18, no. 10: 2099. https://doi.org/10.3390/ijms18102099
APA StylePenzkofer, A., Scheib, U., Stehfest, K., & Hegemann, P. (2017). Absorption and Emission Spectroscopic Investigation of Thermal Dynamics and Photo-Dynamics of the Rhodopsin Domain of the Rhodopsin-Guanylyl Cyclase from the Nematophagous Fungus Catenaria anguillulae. International Journal of Molecular Sciences, 18(10), 2099. https://doi.org/10.3390/ijms18102099