3,5,6,7,8,3′,4′-Heptamethoxyflavone Ameliorates Depressive-Like Behavior and Hippocampal Neurochemical Changes in Chronic Unpredictable Mild Stressed Mice by Regulating the Brain-Derived Neurotrophic Factor: Requirement for ERK Activation
Abstract
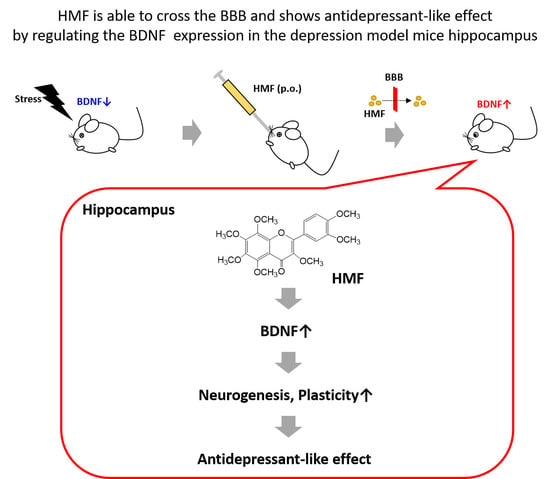
:1. Introduction
2. Results
2.1. Assessment of Brain Levels of 3,5,6,7,8,3′,4′-Heptamethoxyflavone (HMF) after Its Oral Administration
2.2. Effects of Chronic Unpredictable Mild Stress (CUMS) and HMF Treatments on Pathological Conditions
2.3. Effects of HMF on Depressive-Like Behavior in CUMS-Treated Mice
2.4. Effects of CUMS and HMF Treatments on Hippocampal BDNF Protein Expression
2.5. Effects of CUMS and HMF Treatments on Hippocampal Neurogenesis
2.6. Effects of CUMS and HMF Treatments on the Levels of Phosphorylated Calcium-Calmodulin-Dependent Protein Kinase (p-CaMK) II
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Brain Sample Preparation for the LC/MS Analysis
4.3. Quantitative Analysis of HMF via LC/MS
4.4. CUMS Procedure
4.5. Drug Treatment of CUMS-Treated Mice
4.6. FST
4.7. Measurement of Serum CORT Concentrations
4.8. Western Blot Analysis
4.9. Immunohistochemistry
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barde, Y.A. Trophic factors and neuronal survival. Neuron 1989, 2, 1525–1534. [Google Scholar] [CrossRef]
- Angelucci, F.; Brenè, S.; Mathé, A.A. BDNF in schizophrenia, depression and corresponding animal models. Mol. Psychiatry 2005, 10, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Björkholm, C.; Monteggia, L.M. BDNF: A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Knorr, U.; Vinberg, M.; Kessing, L.V.; Wetterslev, J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology 2010, 35, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S. Neurotrophic factors and regulation of mood: Role of exercise, diet and metabolism. Neurobiol. Aging 2005, 26, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.B.; Frey, B.N.; Andreazza, A.C.; Goi, J.D.; Rosa, A.R.; Gonçalves, C.A.; Santin, A.; Kapczinski, F. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci. Lett. 2006, 398, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Huang, Z.; Zhong, X.M.; Xian, Y.F.; Ip, S.P. Piperine reverses the effects of corticosterone on behavior and hippocampal BDNF expression in mice. Neurochem. Int. 2014, 74, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Demuyser, T.; Bentea, E.; Deneyer, L.; Albertini, G.; Massie, A.; Smolders, I. Disruption of the HPA-axis through corticosterone-release pellets induces robust depressive-like behavior and reduced BDNF levels in mice. Neurosci. Lett. 2016, 626, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, C.; Yalcin, E.S.; Aksaray, S.; Kisa, C.; Yildirim, S.G.; Uzbay, T.; Goka, E. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y.; Rao, J.S.; Rizavi, H.S.; Kotowski, J.; Conley, R.R.; Roberts, R.C.; Tamminga, C.A.; Pandey, G.N. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch. Gen. Psychiatry 2003, 60, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Gourley, S.L.; Wu, F.J.; Taylor, J.R. Corticosterone regulates pERK1/2 Map kinase in a chronic depression model. Ann. N. Y. Acad. Sci. 2008, 1148, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Iinuma, M.; Soumiya, H.; Fukumitsu, H.; Furukawa, Y.; Furukawa, S. A novel 2-decenoic acid thioester ameliorates corticosterone-induced depression- and anxiety-like behaviors and normalizes reduced hippocampal signal transduction in treated mice. Pharmacol. Res. Perspect. 2015, 3, e00132. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, A.; Okuyama, S.; Yamamoto, K.; Amakura, Y.; Yoshimura, M.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a citrus flavonoid, ameliorates corticosterone-induced depression-like behavior and restores brain-derived neurotrophic factor expression, neurogenesis, and neuroplasticity in the hippocampus. Molecules 2016, 21, 541. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Miyazaki, K.; Yamada, R.; Amakura, Y.; Yoshimura, M.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Permeation of polymethoxyflavones into the mouse brain and their effect on MK-801-induced locomotive hyperactivity. Int. J. Mol. Sci. 2017, 18, 489. [Google Scholar] [CrossRef] [PubMed]
- Willner, P.; Towell, A.; Sampson, D.; Sophokleous, S.; Muscat, R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 1987, 93, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Monleon, S.; Parra, A.; Simon, V.M.; Brain, P.F.; D’Aquila, P.; Willner, P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology 1995, 117, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Grønli, J.; Bramham, C.; Murison, R.; Kanhema, T.; Fiske, E.; Bjorvatn, B.; Ursin, R.; Portas, C.M. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol. Biochem. Behav. 2006, 85, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Tianzhu, Z.; Shihai, Y.; Juan, D. Antidepressant-like effects of cordycepin in a mice model of chronic unpredictable mild stress. Evid. Based Complement. Altern. Med. 2014, 2014, 438506. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Okuyama, S.; Amakura, Y.; Watanabe, S.; Fukata, T.; Nakajima, M.; Yoshimura, M.; Yoshida, T. Isolation and characterization of activators of ERK/MAPK from citrus plants. Int. J. Mol. Sci. 2012, 13, 1832–1845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Jin, H.; Lin, H.B.; Yang, X.M.; Cheng, Y.F.; Deng, F.J.; Xu, J.P. Antidepressant effect of the extracts from Fructus Akebiae. Pharmacol. Biochem. Behav. 2010, 94, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.F.; Nie, Y.X.; Tong, Q.Z.; Tang, Y.L.; Zeng, Y.; Jing, K.Q.; Zheng, X.; Liao, D.F. Epigallocatechin-3-gallate attenuates impairment of learning and memory in chronic unpredictable mild stress-treated rats by restoring hippocampal autophagic flux. PLoS ONE 2014, 9, e112683. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology 2004, 177, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, P.J.; Oomen, C.A.; Schouten, M.; Encinas, J.M.; Fitzsimons, C.P. Adult neurogenesis, chronic stress and depression. In Adult Neurogenesis in the Hippocampus: Health, Psychopathology, and Brain Disease; Juan, J.C., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 177–206. [Google Scholar]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Davila, D.; Thibault, K.; Fiacco, T.A.; Agulhon, C. Recent molecular approaches to understanding astrocyte function in vivo. Front. Cell. Neurosci. 2013, 24, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Quesseveur, G.; David, D.J.; Gaillard, M.C.; Pla, P.; Wu, M.V.; Nguyen, H.T.; Nicolas, V.; Auregan, G.; David, I.; Dranovsky, A.; et al. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl. Psychiatry 2013, 3, e253. [Google Scholar] [CrossRef] [PubMed]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [PubMed]
- Paizanis, E.; Hamon, M.; Lanfumey, L. Hippocampal neurogenesis, depressive disorders, and antidepressant therapy. Neural Plast. 2007, 2007, 7. [Google Scholar] [CrossRef] [PubMed]
- Vithlani, M.; Hines, R.M.; Zhong, P.; Terunuma, M.; Hines, D.J.; Revilla-Sanchez, R.; Jurd, R.; Haydon, P.; Rios, M.; Brandon, N.; et al. The ability of BDNF to modify neurogenesis and depressive-like behaviors is dependent upon phosphorylation of tyrosine residues 365/367 in the GABAA-receptor γ2 subunit. J. Neurosci. 2013, 33, 15567–15577. [Google Scholar] [CrossRef] [PubMed]
- Gerges, N.Z.; Aleisa, A.M.; Schwarz, L.A.; Alkadhi, K.A. Reduced basal CAMKII levels in hippocampal CA1 region: Possible cause of stress induced impairment of LTP in chronically stressed rats. Hippocampus 2004, 14, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yuan, Y.; Xu, Z.; Pu, M.; Wang, C.; Zhang, Y.; Liu, Z.; Wang, C.; Li, L.; Zhang, Z. Genetic variation in the calcium/calmodulin-dependent protein kinase (CaMK) pathway is associated with antidepressant response in females. J. Affect. Disord. 2012, 136, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, C.; Brambilla, R. Ras-related and MAPK signalling in neuronal plasticity and memory formation. Cell. Mol. Life Sci. 2000, 57, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Wong, J. Neurotrophin signaling and Alzheimer’s disease neurodegeneration-focus on BDNF/TrkB signaling. In Trends in Cell Signaling Pathways in Neuronal Fate Decision; Wislet-Gendebien, S., Ed.; In Tech: Munich, Germany, 2013; pp. 181–194. [Google Scholar]
- Bhatt, D.K.; Ramachandran, R.; Christensen, S.L.; Gupta, S.; Jansen-Olesen, I.; Olesen, J. CGRP infusion in unanesthetized rats increases expression of c-Fos in the nucleus tractus solitarius and caudal ventrolateral medulla, but not in the trigeminal nucleus caudalis. Cephalalgia 2015, 35, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Finkbeiner, S.; Amold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ influx regulates BDNF synthesis by a CREB family transcription factor-dependent mechanism. Neuron 1998, 20, 709–726. [Google Scholar] [CrossRef]
- Nagase, H.; Omae, N.; Omori, A.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Yamakuni, T.; Ohizumi, Y. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem. Biophys. Res. Commun. 2005, 337, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Yoshida, M.; Sun, W.; Nakajima, A.; Lai, Y.; Osaka, N.; Matsuzaki, K.; Yokosuka, A.; Mimaki, Y.; Naganuma, A.; et al. Potent activity of nobiletin-rich Citrus reticulata peel extract to facilitate cAMP/PKA/ERK/CREB signaling associated with learning and memory in cultured hippocampal neurons: Identification of the substances responsible for the pharmacological action. J. Neural Transm. 2013, 120, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, X.L.; Li, J.; Mu, R.H.; Li, Q.; Yi, L.T.; Geng, D. Macranthol promotes hippocampal neuronal proliferation in mice via BDNF-TrkB-PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2015, 762, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Corrêa da Costa, V.M.; Mousovich-Neto, F. Chronic Stress: Glucocorticoids and Metabolic Disturbances. J. Clin. Mol. Endocrinol. 2016, 1, 34–35. [Google Scholar]
- Zhou, W.; Yoshioka, M.; Yokogoshi, H. Sub-chronic effects of s-limonene on brain neurotransmitter levels and behavior of rats. J. Nutr. Sci. Vitaminol. 2009, 55, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Shimada, N.; Kaji, M.; Morita, M.; Miyoshi, K.; Minami, S.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Watanabe, S.; et al. Heptamethoxyflavone, a citrus flavonoid, enhances brain-derived neurotrophic factor production and neurogenesis in the hippocampus following cerebral global ischemia in mice. Neurosci. Lett. 2012, 528, 190–195. [Google Scholar] [CrossRef] [PubMed]










| Day | Stress | Duration |
|---|---|---|
| 1 | Forced swim | 15 min |
| 2 | Damp sawdust | 24 h |
| 3 | Food deprivation | 24 h |
| 4 | Restraint stress | 2 h |
| 5 | Cage tilt | 24 h |
| 6 | Electric footshock | 0.5 mA, 10 s duration, 30 s interval, 20 shocks |
| 7 | Damp sawdust | 24 h |
| 8 | Restraint stress | 2 h |
| 9 | Food deprivation | 24 h |
| 10 | Cage tilt | 24 h |
| 11 | Electric footshock | 0.5 mA, 10 s duration, 30 s interval, 20 shocks |
| 12 | Food deprivation | 24 h |
| 13 | Restraint stress | 2 h |
| 14 | Cage tilt | 24 h |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawamoto, A.; Okuyama, S.; Amakura, Y.; Yoshimura, M.; Yamada, T.; Yokogoshi, H.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone Ameliorates Depressive-Like Behavior and Hippocampal Neurochemical Changes in Chronic Unpredictable Mild Stressed Mice by Regulating the Brain-Derived Neurotrophic Factor: Requirement for ERK Activation. Int. J. Mol. Sci. 2017, 18, 2133. https://doi.org/10.3390/ijms18102133
Sawamoto A, Okuyama S, Amakura Y, Yoshimura M, Yamada T, Yokogoshi H, Nakajima M, Furukawa Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone Ameliorates Depressive-Like Behavior and Hippocampal Neurochemical Changes in Chronic Unpredictable Mild Stressed Mice by Regulating the Brain-Derived Neurotrophic Factor: Requirement for ERK Activation. International Journal of Molecular Sciences. 2017; 18(10):2133. https://doi.org/10.3390/ijms18102133
Chicago/Turabian StyleSawamoto, Atsushi, Satoshi Okuyama, Yoshiaki Amakura, Morio Yoshimura, Takashi Yamada, Hidehiko Yokogoshi, Mitsunari Nakajima, and Yoshiko Furukawa. 2017. "3,5,6,7,8,3′,4′-Heptamethoxyflavone Ameliorates Depressive-Like Behavior and Hippocampal Neurochemical Changes in Chronic Unpredictable Mild Stressed Mice by Regulating the Brain-Derived Neurotrophic Factor: Requirement for ERK Activation" International Journal of Molecular Sciences 18, no. 10: 2133. https://doi.org/10.3390/ijms18102133
APA StyleSawamoto, A., Okuyama, S., Amakura, Y., Yoshimura, M., Yamada, T., Yokogoshi, H., Nakajima, M., & Furukawa, Y. (2017). 3,5,6,7,8,3′,4′-Heptamethoxyflavone Ameliorates Depressive-Like Behavior and Hippocampal Neurochemical Changes in Chronic Unpredictable Mild Stressed Mice by Regulating the Brain-Derived Neurotrophic Factor: Requirement for ERK Activation. International Journal of Molecular Sciences, 18(10), 2133. https://doi.org/10.3390/ijms18102133