CDX2 Stimulates the Proliferation of Porcine Intestinal Epithelial Cells by Activating the mTORC1 and Wnt/β-Catenin Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. Overexpression of CDX2 in IPEC-J2 Cells Increased Cell Proliferation
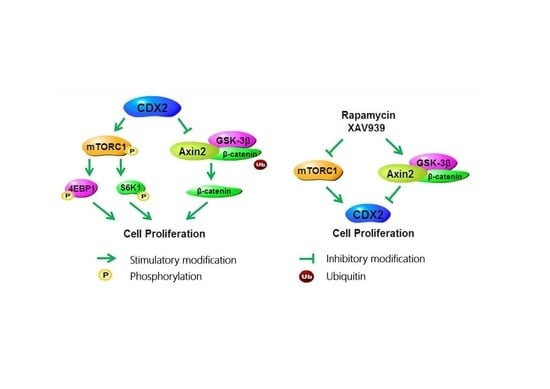
2.2. CDX2 Overexpression Activated Both the mTORC1 and Wnt/β-Catenin Pathways in IPEC-J2 Cells
2.3. CDX2 Knockdown in IPEC-J2 Cells Decreased Cell Proliferation
2.4. CDX2 Knockdown Inhibited Both the mTORC1 and Wnt/β-Catenin Pathways in IPEC-J2 Cells
2.5. Specific Antagonists Decreased Cell Proliferation and the Protein Level of CDX2 in CDX2 Overexpressing IPEC-J2 Cells
2.6. Specific Antagonists Inhibited Both the mTORC1 and Wnt/β-Catenin Pathways in CDX2 Overexpressing IPEC-J2 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. RNA Interference
4.3. Immunofluorescence Microscopy
4.4. Real-Time PCR
4.5. MTT Assay
4.6. Cell Counting Assay
4.7. Western Blot Analysis
4.8. Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CDX2 | caudal type homeobox 2 |
| mTORC1 | mammalian target of rapamycin complex 1 |
| IPEC-J2 | porcine jejunum epithelial cell line |
| ISCs | intestinal stem cells |
| EGF | epidermal growth factor |
| PCNA | proliferation cell nuclear antigen |
| siRNA | small interfering RNA |
| Axin2 | axis inhibition protein 2 |
| GSK-3β | glycogen synthase kinase 3β |
| eIF4E | eukaryotic initiation factor 4E |
| S6K1 | ribosomal protein S6 Kinase-1 |
| 4EBP1 | eukaryotic initiation factor 4E binding protein 1 |
| VCs | villus cells |
| HIEC | human intestinal epithelial crypt cell |
References
- Stringer, E.J.; Duluc, I.; Saandi, T.; Davidson, I.; Bialecka, M.; Sato, T.; Barker, N.; Clevers, H.; Pritchard, C.A.; Winton, D.J.; et al. CDX2 determines the fate of postnatal intestinal endoderm. Development 2012, 139, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Simmini, S.; Bialecka, M.; Huch, M.; Kester, L.; van de Wetering, M.; Sato, T.; Beck, F.; van Oudenaarden, A.; Clevers, H.; Deschamps, J. Transformation of intestinal stem cells into gastric stem cells on loss of transcription factor CDX2. Nat. Commun. 2014, 5, 5728–5737. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M.; Troelsen, J.T.; Nielsen, O.H. The role of CDX2 in intestinal homeostasis and inflammation. BBA Mol. Basis Dis. 2011, 1812, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.J.; Suh, E.R.; Lynch, J.P. The role of CDX proteins in intestinal development and cancer. Cancer Biol. Ther. 2004, 7, 593–601. [Google Scholar] [CrossRef]
- Hinoi, T.; Loda, M.; Fearon, E.R. Silencing of CDX2 expression in colon cancer via a dominant repression pathway. J. Biol. Chem. 2003, 278, 44608–44616. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.G.; Ye, Y.J.; Shen, D.H.; Lu, Y.Y.; Zhang, Z.T.; Wang, S. PTEN expression and suppression of proliferation are associated with CDX2 overexpression in gastric cancer cells. Int. J. Oncol. 2013, 42, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, T.; Lu, H.M.; Katoh, O.; Watanabe, H. Heparin-binding EGF-like growth factor gene transcription regulated by CDX2 in the intestinal epithelium. Am. J. Physiol. 2002, 283, G840–G847. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Xu, G.F.; Zhai, Z.Y.; Gao, C.Q.; Yan, H.C.; Xi, Q.Y.; Guan, W.T.; Wang, S.B.; Wang, X.Q. CDX2 increases SLC7A7 expression and proliferation of pig intestinal epithelial cells. Oncotarget 2016, 21, 30597–30609. [Google Scholar] [CrossRef] [PubMed]
- Bou, G.; Liu, S.; Sun, M.; Zhu, J.; Xue, B.; Guo, J.; Zhao, Y.M.; Qu, B.; Weng, X.G.; Wei, Y.C.; et al. CDX2 is essential for cell proliferation and polarity in porcine blastocysts. Development 2017, 144, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.K.S.; Tovaglieri, A.; Breault, D.T.; Shivdasani, R.A. Distinct processes and transcriptional targets underlie CDX2 requirements in intestinal stem cells and differentiated villus cells. Stem Cell Rep. 2015, 5, 673–681. [Google Scholar]
- Xu, L.; Cui, W.H.; Zhou, W.C.; Li, D.L.; Li, L.C.; Zhao, P.; Mo, X.T.; Zhang, Z.; Gao, J. Activation of Wnt/β-catenin signalling is required for TGF-β/Smad2/3 signalling during myofibroblast proliferation. J. Cell. Mol. Med. 2017, 21, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.L.; Gao, C.Q.; Li, X.G.; Jin, C.L.; Wang, D.; Shu, G.; Wang, W.C.; Kong, X.F.; Yao, K.; Yan, H.C.; et al. EAAT3 promotes amino acid transport and proliferation of porcine intestinal epithelial cells. Oncotarget 2016, 7, 38681–38692. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.J.; Funakoshi, S.; Lee, H.H.; Kong, J.P.; Lynch, J.P. The intestine-specific transcription factor CDX2 inhibits β-catenin/TCF transcriptional activity by disrupting the β-catenin-TCF protein complex. Carcinogenesis 2010, 31, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Savory, J.G.A.; Bouchard, N.; Pierre, V.; Rijli, F.M.; de Repentigny, Y.; Kothary, R.; Lohnes, D. CDX2 regulation of posterior development through non-Hox targets. Development 2009, 136, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.H.; Wei, W.Y.; Cao, W.L.; Zhang, X.S.; Xie, Y.B.; Xiao, Q. Overexpression of CDX2 in gastric cancer cells promotes the development of multidrug resistance. Am. J. Cancer Res. 2015, 5, 321–332. [Google Scholar] [PubMed]
- Bari, M.F.; Brown, H.; Nicholson, A.G.; Kerr, K.M.; Gosney, J.R.; Wallace, W.A.; Soomro, I.; Muller, S.; Peat, D.; Moore, J.D.; et al. BAI3, CDX2 and VIL1: A panel of three antibodies to distinguish small cell from large cell neuroendocrine lung carcinomas. Histopathology 2014, 64, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Zhang, Y.; Li, F.Q.; Du, X.L.; Zhang, J.P. CDX2 inhibits pancreatic adenocarcinoma cell proliferation via promoting tumor suppressor miR-615–5p. Tumor Biol. 2016, 37, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.C.; Wang, J.; Cai, Z.H.; Zhang, Q.H.; Cai, Z.X.; Wu, J.H. Association between vitamin D receptor gene CDX2 polymorphism and breast cancer susceptibility. Tumor Biol. 2013, 34, 3437–3441. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, M.Y.; Sun, J.J.; Ye, R.S.; Cheng, X.; Sun, R.P.; Wei, L.M.; Li, M.; Lin, D.L.; Jiang, Q.Y.; et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci. Rep. UK 2016, 6, 33862–33873. [Google Scholar] [CrossRef] [PubMed]
- Benoit, Y.D.; Pare, F.; Francoeur, C.; Jean, D.; Tremblay, E.; Boudreau, F.; Escaffit, F.; Beaulieu, J.F. Cooperation between HNF-1 α, CDX2, and GATA-4 in initiating an enterocytic differentiation program in a normal human intestinal epithelial progenitor cell line. Am. J. Physiol. 2010, 298, G504–G517. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.; Hansen, M.; Jensen, T.G.K.; Perearnau, A.; Olsen, A.K.; Bram, L.L.; Bak, M.; Tommerup, N.; Olsen, J.; Troelsen, J.T. Genome-wide analysis of CDX2 binding in intestinal epithelial cells (Caco-2). J. Biol. Chem. 2010, 285, 25115–25125. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, S.; Kong, J.; Crissey, M.A.; Dang, L.; Dang, D.; Lynch, J.P. Intestine-specific transcription factor CDX2 induces E-cadherin function by enhancing the trafficking of E-cadherin to the cell membrane. Am. J. Physiol. 2010, 299, G1054–G1067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Chen, Y.; Ye, Y.; Wang, J.; Wang, H.; Yuan, G.H.; Lin, Z.; Wu, Y.H.; Zhang, Y.; Lin, X.H. Wnt signaling promotes hindgut fate commitment through regulating multi-lineage genes during hESC differentiation. Cell Signal. 2017, 29, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Ouyang, H.; Zhu, T.Q.; Lindvall, C.; Wang, Y.; Zhang, X.J.; Yang, Q.; Bennett, C.; Harada, Y.; Stankunas, K.; et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 2006, 126, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Fujishita, T.; Aoki, K.; Lane, H.A.; Aoki, M.; Taketo, M.M. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in Apc (Delta 716) mice. Proc. Natl. Acad. Sci. USA 2008, 105, 13544–13549. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Turnquist, H.R.; Raimondi, G. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 2009, 9, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.B.; Fan, X.J.; Jiao, Y.; Ban, K.C. Mammalian target of rapamycin regulates expression of β-catenin in hepatocellular carcinoma. Hum. Pathol. 2011, 42, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Sui, W.G.; Gao, C.Q.; Yan, H.C.; Yin, Y.L.; Li, H.C.; Wang, X.Q. l-Glutamate deficiency can trigger proliferation inhibition via down regulation of the mTOR/S6K1 pathway in pig intestinal epithelial cells. J. Anim. Sci. 2016, 94, 1541–1549. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.-B.; Zhai, Z.-Y.; Li, X.-G.; Gao, C.-Q.; Yan, H.-C.; Chen, Z.-S.; Wang, X.-Q. CDX2 Stimulates the Proliferation of Porcine Intestinal Epithelial Cells by Activating the mTORC1 and Wnt/β-Catenin Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 2447. https://doi.org/10.3390/ijms18112447
Fan H-B, Zhai Z-Y, Li X-G, Gao C-Q, Yan H-C, Chen Z-S, Wang X-Q. CDX2 Stimulates the Proliferation of Porcine Intestinal Epithelial Cells by Activating the mTORC1 and Wnt/β-Catenin Signaling Pathways. International Journal of Molecular Sciences. 2017; 18(11):2447. https://doi.org/10.3390/ijms18112447
Chicago/Turabian StyleFan, Hong-Bo, Zhen-Ya Zhai, Xiang-Guang Li, Chun-Qi Gao, Hui-Chao Yan, Zhe-Sheng Chen, and Xiu-Qi Wang. 2017. "CDX2 Stimulates the Proliferation of Porcine Intestinal Epithelial Cells by Activating the mTORC1 and Wnt/β-Catenin Signaling Pathways" International Journal of Molecular Sciences 18, no. 11: 2447. https://doi.org/10.3390/ijms18112447
APA StyleFan, H. -B., Zhai, Z. -Y., Li, X. -G., Gao, C. -Q., Yan, H. -C., Chen, Z. -S., & Wang, X. -Q. (2017). CDX2 Stimulates the Proliferation of Porcine Intestinal Epithelial Cells by Activating the mTORC1 and Wnt/β-Catenin Signaling Pathways. International Journal of Molecular Sciences, 18(11), 2447. https://doi.org/10.3390/ijms18112447