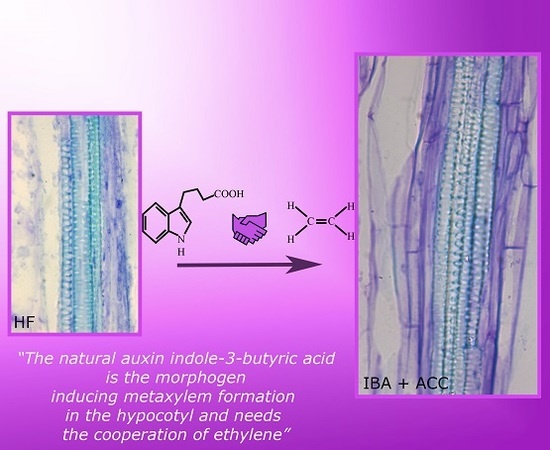
Indole-3-Butyric Acid Induces Ectopic Formation of Metaxylem in the Hypocotyl of Arabidopsis thaliana without Conversion into Indole-3-Acetic Acid and with a Positive Interaction with Ethylene
Abstract
:1. Introduction
2. Results
2.1. The Hypocotyl Pericycle Shows Periclinal Divisions Leading to Ectopic Metaxylem Formation in the Presence of Indole-3-Butyric Acid (IBA)
2.2. The Hypocotyl Pericycle Shows Periclinal Divisions Leading to Both Ectopic Metaxylem and Ectopic Protoxylem in the Presence of 1-Aminocyclopropane-1-Carboxylic Acid (ACC)
2.3. The Xylogenic Response of the ein3eil1 Double Mutant Supports that Ethylene Signalling Is Necessary for Ectopic Metaxylem Formation
2.4. The Xylogenic Response of the ech2ibr10 Double Mutant Shows that the Conversion of IBA into Indole-3-Acetic Acid (IAA) Is Not Necessary to IBA-Induced Metaxylem Formation
3. Discussion
3.1. IBA Is Determinant for the Equilibrium between Ectopic Metaxylem Development and Adventitious Root (AR) Formation in the Hypocotyl Pericycle
3.2. IAA Is Determinant for the Ectopic Protoxylem Formation
3.3. Ethylene Is Required for the IBA-Induced Promotion of Ectopic Metaxylem
3.4. IBA Is a Morphogen
4. Materials and Methods
4.1. Plant Growth
4.2. Histological Analysis
4.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| AR | adventitious root |
| ARP | adventitious root primordium |
| DAS | days after stratification |
| HF | hormone free |
| IAA | indole-3-acetic acid |
| IBA | indole-3-butyric acid |
References
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Del Duca, S.; Cai, G.; Falasca, G.; Altamura, M.M. Arabidopsis SHR and SCR transcription factors and AUX1 auxin influx carrier control the switch between adventitious rooting and xylogenesis in planta and in in vitro cultured thin cell layers. Ann. Bot. 2015, 115, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Růžička, K.; Ursache, R.; Hejátko, J.; Helariutta, Y. Xylem development—From the cradle to the grave. New Phytol. 2015, 207, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H. Tracheary Element Differentiation. Plant Cell 1997, 9, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Pesquet, E.; Jauneau, A.; Digonnet, C.; Boudet, A.M.; Pichon, M.; Goffner, D. Zinnia elegans: The missing link from in vitro tracheary elements to xylem. Physiol. Plant. 2003, 119, 463–468. [Google Scholar] [CrossRef]
- Miyashima, S.; Sebastian, J.; Lee, J.Y.; Helariutta, Y. Stem cell function during plant vascular development. EMBO J. 2013, 32, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Udagawa, M.; Nishikubo, N.; Horiguchi, G.; Yamaguchi, M.; Ito, J.; Mimura, T.; Fukuda, H.; Demura, T. Transcription switches for protoxylem and metaxylem vessel formation. Gene Dev. 2005, 19, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Carlsbecker, A.; Lee, J.Y.; Roberts, C.J.; Dettmer, J.; Lehesranta, S.; Zhou, J.; Lindgren, O.; Moreno-Risueno, M.A.; Vatén, A.; Thitamadee, S.; et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 2010, 465, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Aloni, R.; Zimmermann, M.H. The control of vessel size and density along the plant axis—A new hypothesis. Differentiation 1983, 24, 203–208. [Google Scholar] [CrossRef]
- Sundberg, B.; Uggla, C.; Tuominen, H. Cambial growth and auxin gradients. In Cell and Molecular Biology of Wood Formation; Savidge, R.A., Barnett, J.R., Napier, R., Eds.; BIOS Scientific Publishers: Oxford, UK, 2000; pp. 169–188. [Google Scholar]
- McCann, M.C.; Domingo, C.; Stacey, N.J.; Milioni, D.; Roberts, K. Tracheary element formation in an in vitro system. In Cell and Molecular Biology of Wood Formation; Savidge, R.A., Barnett, J.R., Napier, R., Eds.; BIOS Scientific Publishers: Oxford, UK, 2000; pp. 457–470. [Google Scholar]
- Falasca, G.; Altamura, M.M. Histological analysis of adventitious rooting in Arabidopsis thaliana (L.) Heynh seedlings. Plant Biosyst. 2003, 137, 265–274. [Google Scholar] [CrossRef]
- Ricci, A.; Rolli, E.; Brunoni, F.; Dramis, L.; Sacco, E.; Fattorini, L.; Ruffoni, B.; Díaz-Sala, C.; Altamura, M.M. 1,3-di(benzo[d]oxazol-5-yl)urea acts as either adventitious rooting adjuvant or xylogenesis enhancer in carob and pine microcuttings depending on the presence/absence of exogenous indole-3-butyric acid. Plant Cell Tissue Organ 2016, 126, 411–427. [Google Scholar] [CrossRef]
- Ursache, R.; Miyashima, S.; Chen, Q.; Vatén, A.; Nakajima, K.; Carlsbecker, A.; Zhao, Y.; Helariutta, Y.; Dettmer, J. Tryptophan-dependent auxin biosynthesis is required for HD-ZIP III-mediated xylem patterning. Development 2014, 141, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Warren Wilson, J.; Keys, W.M.S.; Warren Wilson, P.M.; Roberts, L.W. Effects of auxin on the spatial distribution of cell division and xylogenesis in lettuce pith explants. Protoplasma 1994, 183, 162–181. [Google Scholar] [CrossRef]
- Altamura, M.M.; Capitani, F.; Falasca, G.; Gallelli, A.; Scaramagli, S.; Bueno, M.; Torrigiani, P.; Bagni, N. Morphogenesis in cultured thin layers and pith explants of tobacco. I. Effect of putrescine on cell size, xylogenesis and meristemoid organization. J. Plant Physiol. 1995, 147, 101–106. [Google Scholar] [CrossRef]
- Veloccia, A.; Fattorini, L.; Della Rovere, F.; Sofo, A.; D’Angeli, S.; Betti, C.; Falasca, G.; Altamura, M.M. Ethylene and auxin interaction in the control of adventitious rooting in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 6445–6458. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Veloccia, A.; Della Rovere, F.; D’Angeli, S.; Falasca, G.; Altamura, M.M. Indole-3-butyric acid promotes adventitious rooting in Arabidopsis thaliana thin cell layers by conversion into indole-3-acetic acid and stimulation of anthranilate synthase activity. BMC Plant Biol. 2017, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Iakimova, E.T.; Woltering, E.J. Xylogenesis in zinnia (Zinnia elegans) cell cultures: Unravelling the regulatory steps in a complex developmental programmed cell death event. Planta 2017, 245, 681–705. [Google Scholar] [CrossRef] [PubMed]
- Muday, G.K.; Rahman, A.; Binder, B.M. Auxin and ethylene: Collaborators or competitors? Trends Plant Sci. 2012, 17, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.R.; Pengelly, W.L.; Roberts, L.W. Induction of xylem differentiation in Lactuca by ethylene. Plant Physiol. 1984, 75, 1165–1166, Correction in 76, 849. [Google Scholar] [CrossRef] [PubMed]
- Biondi, S.; Scaramagli, S.; Capitani, F.; Marino, G.; Altamura, M.M.; Torrigiani, P. Ethylene involvement in vegetative bud formation in tobacco thin layers. Protoplasma 1998, 202, 134–144. [Google Scholar] [CrossRef]
- Eklund, L.; Little, C.H. Ethylene evolution, radial growth and carbohydrate concentrations in Abies balsamea shoots ringed with Ethrel. Tree Physiol. 1998, 18, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Eklund, L.; Tiltu, A. Cambial activity in ‘normal’ spruce Picea abies Karst (L.) and snake spruce Picea abies (L.) Karst f. virgata (Jacq.) Rehd. in response to ethylene. J Exp. Bot. 1999, 50, 1489–1493. [Google Scholar] [CrossRef]
- Love, J.; Björklund, S.; Vahala, J.; Hertzberg, M.; Kangasjärvi, J.; Sundberg, B. Ethyleneis an endogenous stimulator of cell division in the cambial meristem of Populus. PNAS 2009, 106, 5984–5989. [Google Scholar] [CrossRef] [PubMed]
- Pesquet, E.; Touminen, H. Ethylene stimulates tracheary element differentiation in Zinnia elegans cell cultures. New Phytol. 2011, 190, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Strader, L.C.; Bartel, B. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Mol. Plant. 2011, 4, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Strader, L.C.; Wheeler, D.L.; Christensen, S.E.; Berens, J.C.; Cohen, J.D.; Rampey, R.A.; Bartel, B. Multiple facets of Arabidopsis seedling development require indole-3-butyric acid-derived auxin. Plant Cell 2011, 23, 984–999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, J.; Wen, C.-K. An alternate route of ethylene receptor signaling. Front. Plant Sci. 2014, 5, 648. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.B.; Van Aken, O.; Morreel, K.; Vandenbroucke, K.; van de Cotte, B.; De Clercq, I.; Chiwocha, S.; Fenske, R.; Prinsen, E.; Boerjan, W.; et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT4E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 2010, 22, 2660–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Rovere, F.; Fattorini, L.; D’Angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M.M. Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of Arabidopsis. Ann. Bot. 2013, 112, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Della Rovere, F.; Fattorini, L.; Ronzan, M.; Falasca, G.; Altamura, M.M. The quiescent centre and the stem cell niche in the adventitious roots of Arabidopsis thaliana. Plant Signal. Behav. 2016, 11, e1176660. [Google Scholar] [CrossRef] [PubMed]
- Dharmawardhana, D.P.; Ellis, B.E.; Carlson, J.E. Characterization of vascular lignification in Arabidopsis thaliana. Can. J. Bot. 1992, 70, 2238–2244. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; Muday, G.K.; Dubrovsky, J.D. Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J. 2008, 55, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Růžička, K.; Ljung, K.; Vanneste, S.; Podhorsk, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheres, B.; Di Laurenzio, L.; Willemsen, V.; Hauser, M.-T.; Janmaat, K.; Weisbeek, P.; Benfey, P.N. Mutations affecting the radial organisation of the Arabidopsis root display specific defects throughout the embryonic axis. Development 1995, 121, 53–62. [Google Scholar]
- Parizot, B.; Laplaze, L.; Ricaud, L.; Boucheron-Dubuisson, E.; Bayle, V.; Bonke, M.; De Smet, I.; Poethig, S.R.; Helariutta, Y.; Haseloff, J.; et al. Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiol. 2008, 146, 140–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, M.; Robert, S.; Kleine-Vehn, J. Auxin: Simply complicated. J. Exp. Bot. 2013, 64, 2565–2577. [Google Scholar] [CrossRef] [PubMed]
- Muraro, D.; Mellor, N.; Pound, M.P.; Help, H.; Lucas, M.; Chopard, J.; Byrne, H.M.; Godin, C.; Hodgman, T.C.; King, J.R.; et al. Integration of hormonal signaling networks and mobile microRNAsis required for vascular patterning in Arabidopsis root. PNAS 2014, 111, 857–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esau, K. Plant Anatomy, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1965; pp. 509–514. [Google Scholar]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- Pesquet, E.; Zhang, B.; Gorzsás, A.; Puhakainen, T.; Serk, H.; Escamez, S.; Barbier, O.; Gerber, L.; Courtois-Moreau, C.; Alatalo, E.; et al. Non-cell-autonomous post-mortem lignification of tracheary elements in Zinnia elegans. Plant Cell 2013, 25, 1314–1328. [Google Scholar] [CrossRef] [PubMed]
- Junghans, U.; Langenfeld-Heyser, R.; Polle, A.; Teichmann, T. Effect of auxin transport inhibitors and ethylene on the wood anatomy of poplar. Plant Biol. 2004, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Benková, E.; Ivanchenko, M.G.; Friml, J.; Shishkova, S.; Dubrovsky, J.G. A morphogenetic trigger: Is there an emerging concept in plant developmental biology? Trends Plant Sci. 2009, 14, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Milhinhos, A.; Miguel, C.M. Hormone interactions in xylem development: A matter of signals. Plant Cell Rep. 2013, 32, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Stepanova, A.N.; Solano, R.; Wisman, E.; Ferrari, S.; Ausubel, F.M.; Ecker, J.R. Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. PNAS 2003, 100, 2992–2997. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattorini, L.; Della Rovere, F.; Andreini, E.; Ronzan, M.; Falasca, G.; Altamura, M.M. Indole-3-Butyric Acid Induces Ectopic Formation of Metaxylem in the Hypocotyl of Arabidopsis thaliana without Conversion into Indole-3-Acetic Acid and with a Positive Interaction with Ethylene. Int. J. Mol. Sci. 2017, 18, 2474. https://doi.org/10.3390/ijms18112474
Fattorini L, Della Rovere F, Andreini E, Ronzan M, Falasca G, Altamura MM. Indole-3-Butyric Acid Induces Ectopic Formation of Metaxylem in the Hypocotyl of Arabidopsis thaliana without Conversion into Indole-3-Acetic Acid and with a Positive Interaction with Ethylene. International Journal of Molecular Sciences. 2017; 18(11):2474. https://doi.org/10.3390/ijms18112474
Chicago/Turabian StyleFattorini, Laura, Federica Della Rovere, Eleonora Andreini, Marilena Ronzan, Giuseppina Falasca, and Maria Maddalena Altamura. 2017. "Indole-3-Butyric Acid Induces Ectopic Formation of Metaxylem in the Hypocotyl of Arabidopsis thaliana without Conversion into Indole-3-Acetic Acid and with a Positive Interaction with Ethylene" International Journal of Molecular Sciences 18, no. 11: 2474. https://doi.org/10.3390/ijms18112474
APA StyleFattorini, L., Della Rovere, F., Andreini, E., Ronzan, M., Falasca, G., & Altamura, M. M. (2017). Indole-3-Butyric Acid Induces Ectopic Formation of Metaxylem in the Hypocotyl of Arabidopsis thaliana without Conversion into Indole-3-Acetic Acid and with a Positive Interaction with Ethylene. International Journal of Molecular Sciences, 18(11), 2474. https://doi.org/10.3390/ijms18112474