1. Introduction
Substantial advances have been made over the last two decades in the field of biodegradable polymers, mostly derived from renewable natural resources, to produce bioplastics with features similar to those typical of oil-based materials [
1]. In particular, protein-based edible films and coatings have attracted an increasing interest in recent years since they might be used to protect pharmaceuticals or improve the shelf life of different food products [
2]. These biomaterials are generally first evaluated for their mechanical and barrier properties as a function of different types and concentrations of plasticizers, generally small and nonvolatile organic additives used to increase film extensibility and reduce its cristallinity, brittleness, and water vapor permeability. Plasticizers normally act by decreasing the intermolecular forces along the polymer chains, thus reducing the relative number of polymer–polymer contacts and producing a decrease in the cohesion and tensile strength, thereby increasing the flexibility of the film by allowing its deformation without rupture [
3,
4,
5]. Consequently, plasticizers generally improve the processability of the different biomaterials. Therefore, as the bioplastic industry is continuously growing, the demand for new kinds of plasticizers endowed with specific characteristics and performances compatible with each single bioplastic is growing in parallel [
4,
5].
In the attempt to refine the mechanical and barrier properties of hydrocolloid biopolymers, the effect of the aliphatic polyamines spermidine (SPD) and spermine on both polysaccharide- and protein-based films was recently investigated [
6,
7]. Polyamines are low-molecular-weight polycations, widely distributed in nature and able to mimic the action of divalent ions like Ca
2+ both in vitro and in vivo [
8]. Although the role of polyamines has been associated with aging, various metabolic disorders, cell growth, and cancer, their precise biochemical function is one of the remaining mysteries of molecular cell biology [
9]. The very low toxicity of SPD, attested by an acute oral toxicity of 600 mg/kg in rats [
10] and by an LD50 value higher than 2000 mg/kg in mice [
11], allows its possible addition to edible film-forming solutions (FFSs) to obtain safe food coatings. Thus, SPD effect was tested by including the polyamine in FFSs containing a protein concentrate obtained from bitter vetch (
Vicia ervilia; BV) seeds and different amounts of glycerol (GLY), added as plasticizer [
7]. BV, an annual legume species of the
Vicia genus, is widely cultivated only for livestock feed in temperate regions of Europe, western and central Asia, north Africa, and Americas [
12,
13,
14,
15]. BV seeds, containing up to 25% of protein, were demonstrated to be a potentially useful source for food packaging applications, being not only an abundant protein source but also an inexpensive one for edible film production [
15,
16,
17,
18]. It was recently observed [
7] that films made with a BV protein concentrate (BVPC) in the absence of high concentrations of GLY are brittle, difficult to manipulate, and, consequently, impossible to study. Moreover, the addition of high amounts of GLY were shown unable to give rise to handleable films even when BV proteins were previously denatured by heat treatment at 80 °C for 30 min. Conversely, the presence of appropriate SPD amounts allowed to obtain easily manipulable films at low GLY concentrations and even in the absence of GLY. Therefore, a plasticizing-like effect and a facilitating action on GLY plasticizing effect has been hypothesized for this aliphatic polycation [
7]. In this paper, we report SPD influence on the morphological, mechanical, and barrier properties of films prepared from both native and heat-denatured BV proteins at different concentrations of GLY and pH values.
2. Results and Discussion
Since the addition of millimolar concentrations of SPD to BVPC FFSs allows the formation of handleable films even in the absence of GLY [
7], we were first stimulated to determine the mechanical properties of these GLY-unplasticized films, as well of those prepared in the presence of different amounts of SPD (2–5 mM, corresponding to about 4–9%
w/
w BV protein) and increasing GLY concentrations (4–42 mM GLY, corresponding to about 5–50%
w/
w BV protein). To investigate the influence of SPD and GLY on both native and denatured protein-based films, a BVPC pretreated for 30 min at either 25 or 80 °C was used as a film protein source. Furthermore, to study the influence of the positively charged amino groups of SPD in structuring the film protein network and the consequent film mechanical properties, tensile strength (TS), elongation at break (EB), and Young’s module (YM) values detected for films prepared at pH 8.0 (i.e., under SPD amino group p
Ka values) were compared to those detected for films obtained at pH 11.0 (i.e., over SPD amino group p
Ka values) [
19].
The data reported in
Table 1 indicate that all films made at pH 8.0 with both native and heat-denatured BV proteins and containing SPD alone or with low amounts of GLY showed higher TS, with a maximum value of 12.00 ± 1.21 MPa observed when 3 mM SPD was used in the heat-denatured BVPC FFSs. In addition, the film TS appeared to be progressively lower in all samples containing SPD and increasing GLY concentrations, reaching a very low value (less than 1.0 MPa) at 42 mM GLY. It is worth noting that these latter values are significantly lower than those detected by analyzing films prepared in the presence of GLY alone at high concentrations, thus confirming a positive influence of SPD on GLY plasticizing action.
Consequential and predictable effects were recorded when the EB of the same films was measured (
Table 2). In fact, the lowest EB values were detected when analyzing the films prepared from both native and heat-denatured BVPC in the presence of SPD without or with low concentrations of GLY. Also in this case, EB was observed to rise in parallel with the increase of GLY amount contained in the FFSs where SPD was also present, reaching the maximum values (EB > 80%) at the highest GLY concentration (42 mM). Also such values resulted significantly different (much higher) than those detected when the films were prepared in the presence of high GLY concentrations (33 and 42 mM) but in the absence of SPD.
The same behavior was recorded when measuring the YM of these films, with very high YM values detected for films containing SPD alone (more than 400 MPa) or with low GLY amounts. Also, the YM of SPD-containing films was observed to progressively decrease to very low values (less than 10 MPa) with increasing GLY concentrations up to 42 mM (
Table 3). Also in this case, the YM of films containing both SPD and high amounts of GLY resulted much lower than the YM values observed for films prepared in the presence of high concentrations of GLY but in the absence of SPD.
All these data support our hypothesis on the ability of SPD not only to act as a plasticizer itself by ionically interacting at pH 8.0 with the negative charges occurring onto BV proteins, but also to facilitate in this way GLY action in reducing the intermolecular forces along the protein chains, and consequently to further improve film flexibility and extensibility. This assumption was confirmed by the data of the mechanical properties of the films prepared in the presence of SPD at pH 11.0 (i.e., when its amino groups are uncharged being over their respective p
Ka values) in comparison with those obtained for the films prepared at pH 8.0 (i.e., when SPD is fully protonated). In fact,
Figure 1 clearly indicates that films derived from native BV proteins have different TS, EB, and YM when they are prepared at pH 8.0 or 11.0 in the presence of 3 mM SPD and low GLY concentrations. In particular, the films obtained at pH 8.0 exhibited lower TS and YM and higher EB compared to those prepared at pH 11.0, thus indicating that the different kind of interaction between the polyamine and the folded protein chains is responsible for the different effects on the film mechanical properties. These differences have been proved not to be significant when heat-denatured BV protein films were tested, probably because the unfolded biopolymer chains are influenced by the uncharged SPD in the same way as by the protonated polyamine (
Figure 2). In this case, polyamine–protein hydrogen bonds and hydrophobic interactions may be hypothesized.
Lastly, a further demonstration that SPD was able to enhance the plasticizing performance of GLY was given by the significantly lower EB values (52.56 ± 3.90 vs. 94.45 ± 5.26 for native BV protein films; 51.17 ± 4.80 vs. 95.56 ± 7.62 for denatured BV protein films) and higher YM values (34.11 ± 1.66 vs. 27.66 ± 1.16 for native BV protein films; 68.78 ± 3.60 vs. 13.70 ± 2.70 for denatured BV protein films) detected for films prepared at pH 11.0 in the presence of 42 mM GLY and in the absence of SPD, compared to those containing 42 mM GLY and 3 mM SPD.
Since a primary plasticizer is generally defined as a molecule that, when added to a material, makes it softer, more flexible, and easier to be processed, our findings lead to consider SPD as a possible primary plasticizer of protein-based films. In fact, the addition of millimolar concentrations of SPD allowed BV proteins to give rise to handleable materials as a result of an increase of their elongation and softeness. However, SPD can also be considered as a secondary plasticizer, namely, an “extender”, because of its ability to enhance the plasticizing performance even of a well-known primary plasticizer such as glycerol [
20]. Such double behavior of the polyamine as both a primary and a secondary plasticizer seems to be confirmed also by the analysis of the permeability properties of BVPC films obtained in the presence of different concentrations of SPD and GLY. In fact, the data reported in
Table 4 and
Table 5 showed that the addition of increasing SPD concentrations into FFSs caused in the derived films an increase in the barrier properties to gases (CO
2 and O
2) as well as to water vapor (WV) and that the concomitant presence of low GLY concentrations emphasized this effect. Further increases of GLY concentration into the films, however, led to a marked reversal of the positive barrier effect, thus indicating that an excessive film plasticization promoted film permeability to both gases and WV. It is worthy to note that the same effect was observed by testing films prepared with both native and heat-denatured BV proteins.
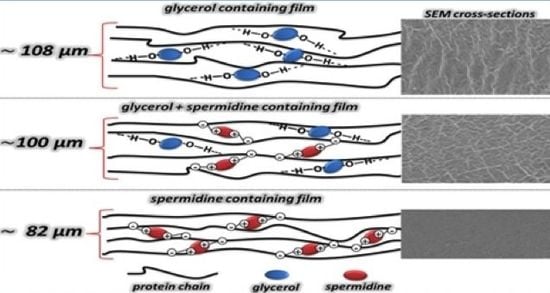
Figure 3 shows the surface and cross-sectional morphology of films derived from FFSs containing native (A–C) or heat denatured BVPC (D,E), mixed with either GLY (A), SPD alone (B,D), or with both plasticizers (C,E). According to the SEM images, BVPC films containing GLY and SPD together presented a relatively smoother and more uniform and continuous appearance with respect to films containing only GLY, which appeared less cohesive and exhibited an evident heterogeneity. Moreover, also the surfaces and the cross sections of the SPD-containing films prepared in the absence of GLY showed features typical of more compact matrices, likely accountable for the lower permeability detected with all films containing the polyamine. These results are in agreement with the marked reduction of thickness of SPD containing films, prepared with both native and denatured BVPC (
Table 6).
Finally, as far as a possible application of SPD/GLY-plasticized films is concerned, we report in
Table 7 the mechanical and barrier properties of various hydrocolloid (polysaccharide- and protein-based) films previously described in the literature, as well as those of some commercial bioplastic (Viscofan NDX and Mater-Bi S-301) and plastic (HD-PE 02) materials analyzed in the present study. The comparison of the features of the SPD-containing BVPC films described here with those of the other hydrocolloid materials indicated similar TS values when BVPC films were prepared in the absence of GLY, whereas higher EB characterized the BVPC films when also GLY was present. Conversely, the barrier effects toward both gases and WV seemed to be similar in all films. More in particular, it is worthy to note that the BVPC films prepared in the presence of low concentrations of both SPD and GLY showed mechanical and barrier characteristics comparable with those of Viscofan NDX [
21], a widely commercialized, collagen-derived edible film largely used in casings for fresh or processed sausages, or dry-cured snacks. Therefore, plant-derived casings made with BVPC and low amounts of both SPD and GLY deserve to be produced and tested as possible alternatives to the casings of animal origin.