Ophthalmic Formulation Containing Nilvadipine Nanoparticles Prevents Retinal Dysfunction in Rats Injected with Streptozotocin
Abstract
:1. Introduction
2. Results
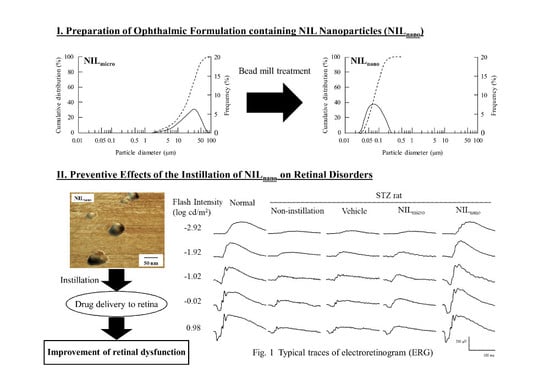
2.1. Retinal Drug Delivery by Ophthalmic Formulations Containing NIL Nanoparticles
2.2. Effects of the Instillation of Nilnano on Retinal Disorders in STZ Rats
3. Discussion
4. Materials and Methods
4.1. Reagents and Animals
4.2. Preparation of Ophthalmic Formulation Containing NIL Nanoparticles
4.3. High Performance Liquid Chromatography (HPLC) Method
4.4. Measurement of Glucose and Insulin Levels in STZ Rats
4.5. Measurement of NIL Concentration in the Blood and Retina
4.6. Measurement of ERG
4.7. Hematoxylin and Eosin (H.E.) Staining of the Retina
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BAC | benzalkonium chloride |
| ERG | electroretinogram |
| H.E. | hematoxylin and eosin |
| HPβCD | 2-hydroxypropyl-β-cyclodextrin |
| HPLC | high performance liquid chromatography |
| MC | methylcellulose |
| NIL | nilvadipine |
| NILnano | ophthalmic formulations containing nilvadipine nanoparticles |
| OP | oscillatory potentials |
| PLGA | poly (dl-lactide-co-glycolide) |
| S.E. | standard error of the mean |
| STZ rat | streptozotocin-induced rat |
| VDCC | voltage-dependent calcium channel |
References
- Bourlais, C.L.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic drug delivery systems—Recent advances. Prog. Retin. Eye Res. 1998, 17, 33–58. [Google Scholar] [CrossRef]
- Ahmed, I.; Patton, T.F. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest. Ophthalmol. Vis. Sci. 1985, 26, 584–587. [Google Scholar] [PubMed]
- Janoria, K.G.; Gunda, S.; Boddu, S.H.; Mitra, A.K. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv. 2007, 4, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J. Drug Target 2011, 19, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Z.; Li, J.; Sun, S.; Weng, Y.; Chen, H. Diclofenac/biodegradable polymer micelles for ocular applications. Nanoscale 2012, 4, 4667–4673. [Google Scholar] [CrossRef] [PubMed]
- Rafie, F.; Javadzadeh, Y.; Javadzadeh, A.R.; Ghavidel, L.A.; Jafari, B.; Moogooee, M.; Davaran, S. In vivo evaluation of novel nanoparticles containing dexa-methasone for ocular drug delivery on rabbit eye. Curr. Eye Res. 2010, 35, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Yoshioka, T.; Lucarelli, M.; Hwang, L.H.; Langer, R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm. Res. 1991, 8, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Terashima, H.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal delivery of indomethacin-loaded PLGA nanoparticles by iontophoresis. Colloids Surf. B Biointerfaces 2011, 88, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Terashima, H.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal delivery of indomethacin using combination of PLGA nanoparticles and iontophoresis in vivo. Colloids Surf. B Biointerfaces 2012, 92, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Watanabe, A.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal permeability of estradiol using combination of PLGA nanoparticles system and iontophoresis. Colloids Surf. B Biointerfaces 2012, 97, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Tanabe, W.; Tanino, T.; Ito, Y.; Okamoto, N.; Shimomura, Y. Effects of ophthalmic formulations containing cilostazol nanoparticles on retinal vasoconstriction in rats injected with Endothelin-1. Pharm. Anal. Acta 2015, 6. [Google Scholar] [CrossRef]
- Nagai, N.; Deguchi, S.; Otake, H.; Hiramatsu, N.; Yamamoto, N. Therapeutic effect of cilostazol ophthalmic nanodispersions on retinal dysfunction in streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2017, 18, 1971. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, M.; Ono, T.; Hiroi, J.; Esumi, K.; Kikuchi, H.; Kumada, S. Comparison of the cardiovascular effect of FR34235, a new dihydropyridine, with other calcium antagonists. J. Cardiovasc. Pharmacol. 1983, 5, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Otori, Y.; Kusaka, S.; Kawasaki, A.; Morimura, H.; Miki, A.; Tano, Y. Protective effect of nilvadipine against glutamate neurotoxicity in purified retinal ganglion cells. Brain Res. 2003, 961, 213–219. [Google Scholar] [CrossRef]
- Uemura, A.; Mizota, A. Retinal concentration and protective effect against retinal ischemia of nilvadipine in rats. Eur. J. Ophthalmol. 2008, 18, 87–93. [Google Scholar] [PubMed]
- Tomita, K.; Araie, M.; Tamaki, Y.; Nagahara, M.; Sugiyama, T. Effects of nilvadipine, a calcium antagonist, on rabbit ocular circulation and optic nerve head circulation in NTG subjects. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1144–1151. [Google Scholar]
- Hara, H.; Toriu, N.; Shimazawa, M. Clinical potential of lomerizine, a Ca2+ channel blocker as an anti-glaucoma drug: Effects on ocular circulation and retinal neuronal damage. Cardiovasc. Drug Rev. 2004, 22, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Toriu, N.; Sasaoka, M.; Shimazasa, M.; Sugiyama, T.; Hara, H. Effects of lomerizine, a novel Ca2+ channel blocker, on the normal and endothelin-1-disturbed circulation in the optic nerve head of rabbits. J. Ocul. Pharmacol. Ther. 2001, 17, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ono, H.; Hashino, M.; Ito, Y.; Okamoto, N.; Shimomura, Y. Improved corneal toxicity and permeability of tranilast by the preparation of ophthalmic formulations containing its nanoparticles. J. Oleo Sci. 2014, 63, 177–186. [Google Scholar] [PubMed]
- Nagai, N.; Ito, Y. Therapeutic effects of gel ointments containing tranilast nanoparticles on paw edema in adjuvant-induced arthritis rats. Biol. Pharm. Bull. 2014, 37, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y. Effect of solid nanoparticle of indomethacin on therapy for rheumatoid arthritis in adjuvant-induced arthritis rat. Biol. Pharm. Bull. 2014, 37, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Ito, Y. Topical therapies for rheumatoid arthritis by gel ointments containing indomethacin nanoparticles in adjuvant-induced arthritis rat. J. Oleo Sci. 2015, 64, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Ito, Y.; Funakami, Y.; Nishikawa, H.; Kawabata, A. Intravenous administration of cilostazol nanoparticles ameliorates acute ischemic stroke in a cerebral ischemia/reperfusion-induced injury model. Int. J. Mol. Sci. 2015, 16, 29329–29344. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Nakazawa, Y.; Ito, Y.; Kanai, K.; Okamoto, N.; Shimomura, Y. A Nanoparticle-based ophthalmic formulation of dexamethasone enhances corneal permeability of the drug and prolongs its corneal residence time. Biol. Pharm. Bull. 2017, 40, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Iwamae, A.; Tanimoto, S.; Yoshiokam, C.; Ito, Y. Pharmacokinetics and antiinflammatory effect of a novel gel system containing ketoprofen solid nanoparticles. Biol. Pharm. Bull. 2015, 38, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Tanino, T.; Ito, Y. Pharmacokinetic studies of gel system containing ibuprofen solid nanoparticles. J. Oleo Sci. 2016, 65, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Mano, Y.; Tanabe, W.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation of disulfiram prolongs corneal residence time of the drug and reduces intraocular pressure. Exp. Eye Res. 2015, 132, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Mano, Y.; Ito, Y. An ophthalmic formulation of disulfiram nanoparticles prolongs drug residence time in lens. Biol. Pharm. Bull. 2016, 39, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Debbasch, C.; Brignole, F.; Pisella, P.J.; Warnet, J.M.; Rat, P.; Baudouin, C. Qua-ternary ammoniums and other preservatives’ contribution in oxidative stressand apoptosis on Chang conjunctival cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 642–652. [Google Scholar]
- Debbasch, C.; Pisella, P.J.; De Saint Jean, M.; Rat, P.; Warnet, J.M.; Baudouin, C. Mitochondrial activity and glutathione injury in apoptosis induced by byunpreserved and preserved beta-blockers on Chang conjunctival cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2525–2533. [Google Scholar]
- Guenoun, J.M.; Baudouin, C.; Rat, P.; Pauly, A.; Briqnole-Baudouin, F. In vitro comparison of cytoprotective and antioxidative effects of latanoprost;travoprost, and bimatoprost on conjunctiva-derived epithelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4594–4599. [Google Scholar] [CrossRef] [PubMed]
- Guenoun, J.M.; Baudouin, C.; Rat, P.; Pauly, A.; Warnet, J.M.; Briqnole Baudouin, F. In vitro study of inflammatory potential and toxicity profile oflatanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Murao, T.; Okamoto, N.; Ito, Y. Comparison of corneal wound healing rates after instillation of commercially available latanoprost and travoprost in rat debrided corneal epithelium. J. Oleo Sci. 2010, 59, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Lee, S.K.; Cristol, S.M.; Lee, D.W.; Seo, K.Y.; Kim, E.K. Impact of short-term exposure of commercial eyedrops preserved with benzalkoniumchloride on precorneal mucin. Mol. Vis. 2006, 12, 415–421. [Google Scholar] [PubMed]
- Nagai, N.; Yoshioka, C.; Tanino, T.; Ito, Y.; Okamoto, N.; Shimomura, Y. Decrease in Corneal Damage due to Benzalkonium Chloride by the Addition of Mannitol into Timolol Maleate Eye Drops. J. Oleo Sci. 2015, 64, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Yoshioka, N.; Kondo, Y.; Takeuchi, T.; Yamashita, H. Catalyticallyactive; magnetically separable, and water-soluble FePt nanoparticles modi-fied with cyclodextrin for aqueous hydrogenation reactions. Green Chem. 2009, 11, 1337–1342. [Google Scholar] [CrossRef]
- Koevary, S.B. Pharmacokinetics of topical ocular drug delivery: Potential uses for the treatment of diseases of the posterior segment and beyond. Curr. Drug Metab. 2003, 4, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Trick, G.L.; Burde, R.M.; Gordon, M.O.; Kilo, C.; Santiago, J.V. Retinocortical conduction time in diabetics with abnormal pattern reversal electroretinograms and visual evoked potentials. Documenta Ophthalmologica 1988, 70, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.E.; Bearse, M.A., Jr.; Schneck, M.E.; Barez, S.; Adams, A.J. Multifocal VEP (mfVEP) reveals abnormal neuronal delays in diabetes. Documenta Ophthalmologica 2010, 121, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Porciatti, V.; Scalia, G.; Caputo, S.; Minnella, A.; Di Leo, M.A.; Ghirlanda, G. Steady-state pattern electroretinogram in insulin-dependent diabetics with no or minimal retinopathy. Documenta Ophthalmologica 1989, 73, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kohzaki, K.; Vingrys, A.J.; Bui, B.V. Early inner retinal dysfunction in streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3595–3604. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, N.; Deguchi, S.; Yoshioka, C.; Otake, H.; Yamamoto, N.; Nagai, N. Evaluation of Retinal Function in Streptozotocin-induced Diabetic Rats by Using the Electroretinography and Immunohistochemistry Methods. Yakugaku Zasshi 2017, 137, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zemel, E.; Miller, B.; Perlman, I. Early retinal damage in experimental diabetes: Electroretinographical and morphological observations. Exp. Eye Res. 2002, 74, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Medical Package Insert of Nilvadipine; Takeda Chemical Industries, Ltd. NAFTDIL: Sarasota, FL, USA, 2003.
- Nagai, N.; Murao, T.; Okamoto, N.; Ito, Y. Kinetic Analysis of the Rate of Corneal Wound Healing in Otsuka Long-Evans Tokushima Fatty Rats, a Model of Type 2 Diabetes Mellitus. J. Oleo Sci. 2010, 59, 441–449. [Google Scholar] [CrossRef] [PubMed]





| Group | Normal Rat | STZ Rat | ||
|---|---|---|---|---|
| Non-Instillation | Vehicle | NILnano | ||
| Distance (µm) | 71.0 ± 3.57 *2,3 | 130.6 ± 5.46 *1 | 132.5 ± 5.14 *1 | 77.7 ± 4.16 *2,3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deguchi, S.; Otake, H.; Nakazawa, Y.; Hiramatsu, N.; Yamamoto, N.; Nagai, N. Ophthalmic Formulation Containing Nilvadipine Nanoparticles Prevents Retinal Dysfunction in Rats Injected with Streptozotocin. Int. J. Mol. Sci. 2017, 18, 2720. https://doi.org/10.3390/ijms18122720
Deguchi S, Otake H, Nakazawa Y, Hiramatsu N, Yamamoto N, Nagai N. Ophthalmic Formulation Containing Nilvadipine Nanoparticles Prevents Retinal Dysfunction in Rats Injected with Streptozotocin. International Journal of Molecular Sciences. 2017; 18(12):2720. https://doi.org/10.3390/ijms18122720
Chicago/Turabian StyleDeguchi, Saori, Hiroko Otake, Yosuke Nakazawa, Noriko Hiramatsu, Naoki Yamamoto, and Noriaki Nagai. 2017. "Ophthalmic Formulation Containing Nilvadipine Nanoparticles Prevents Retinal Dysfunction in Rats Injected with Streptozotocin" International Journal of Molecular Sciences 18, no. 12: 2720. https://doi.org/10.3390/ijms18122720
APA StyleDeguchi, S., Otake, H., Nakazawa, Y., Hiramatsu, N., Yamamoto, N., & Nagai, N. (2017). Ophthalmic Formulation Containing Nilvadipine Nanoparticles Prevents Retinal Dysfunction in Rats Injected with Streptozotocin. International Journal of Molecular Sciences, 18(12), 2720. https://doi.org/10.3390/ijms18122720