Neurogenic Traditional Chinese Medicine as a Promising Strategy for the Treatment of Alzheimer’s Disease
Abstract
:1. Introduction to Neurogenesis
2. Regulatory Mechanisms of Neurogenesis
2.1. Neurotrophic Factors
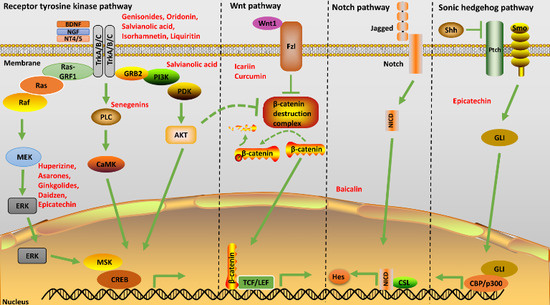
2.2. Signal Transduction
2.2.1. Sonic Hedgehog Pathway
2.2.2. Wnt Pathway
2.2.3. Notch Pathway
2.2.4. Receptor Tyrosine Kinase Pathway
2.3. Miscellaneous Regulators of Neurogenesis
2.3.1. Roles of Epigenetic Modulators in Neurogenesis
2.3.2. Roles of Neurotransmitters in Neurogenesis
3. Neurogenesis and Alzheimer’s Disease
4. Neurogenic Potential of Traditional Chinese Medicine (TCM) Herbs and Its Active Ingredients
4.1. Curcuma longa
4.2. Huperzia serrata
4.3. Panax ginseng
4.4. Salvia miltiorrhiza
4.5. Ginkgo biloba
5. Miscellaneous TCM Active Molecules Showing Neurogenic Potential
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AchE | Acetylcholine esterase |
| AD | Alzheimer’s Disease |
| BDNF | Brain-derived neurotrophic factor |
| CREB | Cyclic adenosine 3,5-monophosphate responsive element binding protein |
| DG | Dentate gyrus |
| GAP43 | Growth associated protein 43 |
| GC | Granule cell |
| GSK3β | Glycogen synthase kinase 3β |
| LDH | Lactate dehydrogenase |
| MAP2 | Microtubule associated protein 2 |
| MAP | Mitogen activated protein kinase |
| NFT | Neurofibrillary tangles |
| NGF | Nerve growth hormone |
| NICD | Notch Intracellular domain |
| NSC | Neural stem cell |
| NT | Neurotrophin |
| OB | Olfactory bulb |
| Ptch | Patched |
| RTK | Receptor tyrosine kinase |
| Shh | Sonic hedgehog |
| Smo | Smoothened |
| SVZ | Sub-Ventricular zone |
| Trk | Tropomyosin-related kinase |
| TCM | Traditional Chinese medicine |
References
- Ming, G.-L.; Hongjun, S. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Lorena, V.-N.; Florencia, A.; Ana, A.; Paula, S.; Nibaldo, I. Adult Hippocampal Neurogenesis in Ageing and Alzheimer’s Disease. Birth Defects Res. 2010, 90, 284–296. [Google Scholar]
- Toni, N.; Matthew, T.; Eric, B.; James, A.; Chunmei, Z.; Antonella, C.; van Henriette, P.; Maryann, M.; Mark, E.; Fred, G. Synapse formation on neurons born in the adult hippocampus. Nat. Neurosci. 2007, 10, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Jae, R.R.; Caroline, H.J.; Joo, K.Y.; Eun, K.K.; Woong, S.; Seong, Y.W. Control of adult neurogenesis by programmed cell death in the mammalian brain. Mol. Brain 2016, 43. [Google Scholar] [CrossRef]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Waterhouse, E.; Xu, B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol. Cell Neurosci. 2009, 42, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.; Reichardt, L. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Duan, W.; Mattson, M. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.; Goodman, J.; Macleod, A.; Phani, S.; Antonelli, C.; Croll, S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurobiol. 2005, 192, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Heidi, P. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 1991, 7, 695–702. [Google Scholar]
- Narisawa-Saito, M.; Wakabayashi, K.; Tsuji, S.; Takahashi, H.; Nawa, H. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer’s disease. Neuroreport 1996, 7, 2925–2928. [Google Scholar] [CrossRef] [PubMed]
- Frielingsdorf, H.; Simpson, D.R.; Thal, L.J.; Pizzo, D.P. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol. Dis. 2007, 26, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Angelucci, A.; Costantin, L.; Braschi, C.; Mazzantini, M.; Babbini, F.; Fabbri, M.E.; Tessarollo, L.; Maffei, L.; Berardi, N.; et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis fol- lowing environmental enrichment. Eur. J. Neurosci. 2006, 24, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, K.; Zhao, M.; Sakata, K.; Akbarian, S.; Bates, B.; Jaenisch, R.; Lu, B. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn. Mem. 2006, 13, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Hagg, T. From Neurotransmitters to Neurotrophic Factors to Neurogenesis. Neuroscientist 2009, 15, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Kaspar, B.; Gage, F.; Schaffer, D. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 2003, 6, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lie, D.C.; Colamarino, S.A.; Song, H.J.; Désiré, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005, 437, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Tata, P.R.; Kuhl, M. An Updated Overview on Wnt Signaling Pathways a Prelude for More. Circ. Res. 2010, 106, 1796–1806. [Google Scholar]
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, C.; Campano, L.M.; Wöhrle, S.; Hecht, A. Canonical Wnt signaling transiently stimulates proliferation and enhances neurogenesis in neonatal neural progenitor cultures. Exp. Cell Res. 2007, 13, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Machon, O.; Backman, M.; Machonova, O.; Kozmik, Z.; Vacik, T.; Andersen, L.; Krauss, S. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev. Biol. 2007, 311, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Jianing, L.; Chihiro, S.; Massimiliano, C.; Amy, W. Notch Signaling in the Regulation of Stem Cell Self-Renewal and Differentiation. Curr. Top. Dev. Biol. 2010, 92, 367–409. [Google Scholar]
- Bolos, V.; Joaquin Grego, B.; Jose Luis, P. Notch signaling in development and cancer. Endocr. Rev. 2007, 28, 339–363. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S. Concise Review: Notch signaling in stem cell regulation. Stem Cells 2006, 24, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Kenichi, M.; Keejung, Y.; Louis, D.; Akinori, T.; Nicholas, G. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 2007, 449, 351–355. [Google Scholar]
- Tarran, P.; Lavinia, A.; Nicholas, G. Notch in the Vertebrate Nervous System: An Old Dog with New Tricks. Neuron 2011, 69, 840–855. [Google Scholar]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Pawson, T. Regulation and targets of receptor tyrosine kinases. Eur. J. Cancer 2002, 38, S3–S10. [Google Scholar] [CrossRef]
- Barbacid, M. The Trk family of neurotrophin receptors. J. Neurobiol. 1994, 25, 1386–1403. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V.; Rajagopal, R.; Lee, F.S. Neurotrophin signalling in health and disease. Clin. Sci. 2006, 110, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Marchetto, M.C.; Guo, J.U.; Ming, G.L.; Gage, F.H.; Song, H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat. Neurosci. 2010, 13, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Daniel, B.; Laure, B.; Hongjun, S.; Andras, S. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 2013, 140, 2548–2561. [Google Scholar]
- Encinas, J.M.; Vaahtokari, A.; Enikolopov, G. Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA 2006, 103, 8233–8238. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Arnold, S.A.; Habas, A.; Hetman, M.; Hagg, T. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J. Neurosci. 2008, 28, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Aguado, T.; Romero, E.; Monory, K.; Palazuelos, J.; Sendtner, M.; Marsicano, G.; Lutz, B.; Guzmán, M.; Galve-Roperh, I. The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neuro- genesis. J. Biol. Chem. 2007, 282, 23892–23898. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, O.; Deveci, A.; Taneli, F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: A preliminary study. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2005, 29, 261–265. [Google Scholar] [CrossRef] [PubMed]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-β in Alzheimer’s disease. Nat. Rev. Neurosci. 2007, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Mufson, E.J. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Ageing 2006, 27, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Ziabreva, I.; Perry, E.; Perry, R.; Minger, S.L.; Ekonomou, A.; Przyborski, S.; Ballard, C. Altered neurogenesis in Alzheimer’s disease. J. Psychosom. Res. 2006, 61, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Winkler, J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, O.; Marr, R.A. Of mice and men: Neurogenesis, cognition and Alzheimer’s disease. Front. Aging Neurosci. 2013, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Taniuchi, N.; Akaike, A.; Kihara, T.; Sugimoto, H. Differential regulation of neurogenesis in two neurogenic regions of APPswe/PS1dE9 transgenic mice. Neuroreport 2008, 19, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, N.; Niidome, T.; Goto, Y.; Akaike, A.; Kihara, T.; Sugimoto, H. Decreased proliferation of hippocampal progenitor cells in APPswe/PS1dE9 transgenic mice. Neuroreport 2007, 18, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.; Verret, L.; Bozon, B.; El Tannir El Tayara, N.; Ly, M.; Kober, F.; Dhenain, M.; Rampon, C.; Delatour, B. Impaired neurogenesis, neuronal loss, and brain functional deficits in the APPxPS1-Ki mouse model of Alzheimer’s disease. Neurobiol. Aging 2011, 32, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Adem, A.; Sabbagh, M. New Acetylcholinesterase Inhibitors for Alzheimer’s Disease. Int. J. Alzheimer Dis. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S.; So, K.-F.; Chuen-Chung Chang, R. Drug discovery from Chinese medicine against neurodegeneration in Alzheimer’s and vascular dementia. Chin. Med. 2011, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Fan, J.; Han, J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm. Sin. B 2015, 5, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Curcumin-Loaded Nanoparticles Potently Induce Adult Neurogenesis and Reverse Cognitive Deficits in Alzheimer’s Disease Model via Canonical Wnt/β-Catenin Pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Li-Li, T.; Rui, W.; Xi-can, T. Huperzine A protects SHSY5Y neuroblastoma cells against oxidative stress damage via nerve growth factor production. Eur. J. Pharmacol. 2005, 519, 9–15. [Google Scholar]
- Liu, X.-Y.; Hou, J.-C.; Zhu, H.; Wong, Z.; Liu, J.-X.; Zheng, Y.-Q. Ginsenoside Rd promotes neurogenesis in rat brain after transient focal cerebral ischemia via activation of PI3K/Akt pathway. Acta Pharmacol. Sin. 2015, 36, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Tchantchou, F.; Lacor, P.; Cao, Z.; Lao, L.; Hou, Y.; Cui, C.; Klein, W.; Luo, Y. Stimulation of Neurogenesis and Synaptogenesis by Bilobalide and Quercetin via Common Final Pathway in Hippocampal Neurons. J. Alzheimer Dis. 2009, 18, 787–798. [Google Scholar]
- Zhang, N.; Kang, T.; Xia, Y.; Wen, Q.; Zhang, X.; Li, H.; Hu, Y.; Hao, H.; Zhao, D.; Sun, D.; et al. Effects of salvianolic acid B on survival, self-renewal and neuronal differentiation of bone marrow derived neural stem cells. Eur. J. Pharmacol. 2012, 697, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Huang, S.; Liu, S.; Feng, X.-L.; Yu, M.; Liu, J.; Sun, Y.-E.; Chen, G.; Yu, Y.; Zhao, J.; et al. A herbal medicine for Alzheimer’s disease and its active constituents promote neural progenitor proliferation. Aging Cell 2015, 14, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Stringer, T.; Guerrieri, D.; Vivar, C.; Praag, H.V. Plant-derived flavanol (−) epicatechin mitigates anxiety in association with elevated hippocampal monoamine and BDNF levels, but does not influence pattern separation in mice. Transl. Psychiatry 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.-Q.; Liu, D.-L.; Wei, W.-T.; Chen, L.; Huang, H.; Li, Y.; Cui, J.-H. Oridonin induces apoptosis in SW1990 pancreatic cancer cells via p53- and caspase-dependent induction of p38 MAPK. Oncol. Rep. 2014, 31, 975–982. [Google Scholar] [PubMed]
- Wang, Z.; Liu, Q.; Zhang, R.; Liu, S.; Xia, Z.; Hu, Y. Catalpol ameliorates β amyloid-induced degeneration of cholinergic neurons by elevating brain-derived neurotrophic factors. Neuroscience 2009, 163, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Jesky, R.; Chen, H. The neuritogenic and neuroprotective potential of senegenin against Aβ-induced neurotoxicity in PC12 cells. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin Structure-Function, Bioavailability, and Efficacy in Models of Neuroinflammation and Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Epigallocatechin-3-gallate and curcumin suppress amyloid β-site APP cleaving enzyme-1 upreglation. Neuroreport 2008, 19, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Kelloff, G.J.; Boone, C.W.; Crowell, J.A.; Nayfield, S.G.; Hawk, E.T.; Steele, V.E.; Lubet, R.A.; Sigman, C.C. Perspectives and progress in development of breast cancer chemopreventive drugs. Environ. Health Perspect. 1997, 396, 159–183. [Google Scholar]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The Curry Spice Curcumin Reduces Oxidative Damage and Amyloid Pathology in an Alzheimer Transgenic Mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [PubMed]
- Laura, Z.; Milan, F.; John, C.; James, S.; Araceli, E.; Michelle, M.; Justin, Z.; Vladimir, B.; Michael, C.G.; George, B.; et al. Curcuminoids enhance amyloid-β uptake by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2006, 10, 1–7. [Google Scholar]
- Dong, S.; Zeng, Q.; Mitchell, E.S.; Xiu, J.; Duan, Y.; Li, C.; Tiwari, J.K.; Hu, Y.; Cao, X.; Zhao, Z. Curcumin Enhances Neurogenesis and Cognition in Aged Rats: Implications for Transcriptional Interactions Related to Growth and Synaptic Plasticity. PLoS ONE 2012, 7, e31211. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ku, B.; Cui, L.; Li, X.; Barish, P.A.; Foster, T.C.; Ogle, W.O. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007, 1162, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ku, B.; Tie, L.; Yao, H.; Jiang, W.; Ma, X.; Li, X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006, 1122, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.L.; Tang, X.C.; He, X.C. Huperzine A, a potential therapeutic agent for treatment of Alzheimer’s disease. Curr. Med. Chem. 2000, 7, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.H.; Ren, H.; Tang, X.C. Huperzine A, a novel promising acetylcholinesterase inhibitor. Neuroreport 1996, 8, 97–101. [Google Scholar]
- Felgenhauer, N.; Zilker, T.; Worek, F.; Eyer, P. Intoxication with Huperzine A, a potent anti-cholinesterase found in the fir club moss. J. Toxicol. Clin. Toxicol. 2000, 38, 803–808. [Google Scholar]
- Ma, X.; Tan, C.; Zhu, D.; Gang, D.; Xiao, P. Huperzine A from Huperzia species—An ethnopharmacolgical review. J. Ethnopharmacol. 2007, 113, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.-L.; Wang, R.; Tang, X.-C. Effects of Huperzine A on secretion of nerve growth factor in cultured rat cortical astrocytes and neurite outgrowth in rat PC12 cells. Acta Pharmacol. Sin. 2005, 26, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.H.; Zhu, C.X.; Zhang, R.; An, L. Huperzine A in the Treatment of Alzheimer’s Disease and Vascular Dementia: A Meta-Analysis. Evid.-Based Compliment. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Gong, K.; Yan, Y.; Zhang, L.; Tang, P.; Zhang, X.; Gong, Y. Huperzine A promotes hippocampal neurogenesis in vitro and in vivo. Brain Res. 2013, 1506, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.; Farooqui, T.; Koh, H.; Farooqui, A.A.; Ling, E. Protective effects of ginseng on neurological disorders. Front. Aging Neurosci. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhou, Q.; Sun, S.; Leung, K.S.; Zhang, H.; Zhao, Z. Differentiation of Asian ginseng, American ginseng and Notoginseng by Fourier transform infrared spectroscopy combined with two-dimensional correlation infrared spectroscopy. J. Mol. Struct. 2008, 883–884, 91–98. [Google Scholar] [CrossRef]
- Xu, F.-Y.; Shang, W.-Q.; Yu, J.-J.; Sun, Q.; Li, M.-Q.; Sun, J.-S. The antitumor activity study of ginsenosides and metabolites in lung cancer cell. Am. J. Transl. Res. 2016, 8, 1708–1718. [Google Scholar] [PubMed]
- Park, H.-W.; In, G.; Han, S.-T.; Lee, M.-W.; Kim, S.-Y.; Kim, K.-T.; Cho, B.-G.; Han, G.-H.; Chang, I.-M. Simultaneous determination of 30 ginsenosides in Panax ginseng preparations using ultra performance liquid chromatography. J. Ginseng Res. 2013, 37, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, P.; Shin, Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J. Ginseng Res. 2013, 37, 8–29. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Li, Y.; Quan, Q.; Wang, J. Cellular and molecular mechanisms underlying the action of ginsenoside Rg1 against Alzheimer’s disease. Neural Regen. Res. 2012, 7, 2860–2866. [Google Scholar] [PubMed]
- Tian, D.-D.; Jia, W.-W.; Wang, D.-D.; Liu, J.-H.; Ding, J.-J.; Li, L.; Du, F.-F.; Xu, F.; Wang, F.-Q.; Sun, Y.; et al. Methylation and its role in the disposition of tanshinol, a cardiovascular carboxylic catechol from Salvia miltiorrhiza roots (Danshen). Acta Pharmacol. Sin. 2015, 36, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Hsieh, C.-L. Pharmacological effects of Salvia miltiorrhiza (Danshen) on cerebral infarction. Chin. Med. 2010, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Durairajan, S.S.K.; Yuan, Q.; Xie, L.; Chan, W.-S.; Kum, W.-F.; Koo, I.; Liu, C.; Song, Y.; Huang, J.-D.; Klein, W.; et al. Salvianolic acid B inhibits Aβ fibril formation and disaggregates preformed fibrils and protects against Aβ-induced cytotoxicity. Neurochem. Int. 2008, 52, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zuo, Z.; Chow, M.S. Danshen: An Overview of Its Chemistry, Pharmacology, Pharmacokinetics, and Clinical Use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; WenBo, L.; XiaoDong, C.; Lei, Z.; Yan, Q.; Jun, H.; Zhou, F. Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Res. Bull. 2011, 84, 163–168. [Google Scholar]
- Young, W.L.; Dong, H.K.; Su, J.J.; Se, J.P.; Jong, M.K.; Jun, M.J.; Hyung, E.L.; Shin, G.B.; Hee, K.O.; Kun, H.H.S.; et al. Neuroprotective effects of salvianolic acid B on an Aβ25–35 peptide-induced mouse model of Alzheimer’s disease. Eur. J. Pharmacol. 2013, 704, 70–77. [Google Scholar]
- Guo, G.; Li, B.; Wang, Y. Effects of salvianolic acid B on proliferation, neurite outgrowth and differentiation of neural stem cells derived from cerebral cortex of embryonic mice. Sci. China Life Sci. 2010, 63, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Shen, J.; Fai So, K. Chinese Traditional Medicine and Adult Neurogenesis in the Hippocampus. J. Tradit. Complement. Med. 2014, 4, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Zhang, Y.; Cui, G.; Bian, Y.; Zhang, M.; Zhang, J. Direct Stimulation of Adult Neural Stem/Progenitor Cells In Vitro and Neurogenesis In Vivo by Salvianolic Acid B. PLoS ONE 2012, 7, e35636. [Google Scholar] [CrossRef] [PubMed]
- Le, B.P. Magnitude of effect and special approach to Ginkgo biloba extract EGb761 in cognitive disorders. Pharmacopsychiatry 2003, 36, S44–S49. [Google Scholar]
- Shi, C.; Zhao, L.; Zhu, B.; Li, Q.; Yew, D.; Yao, Z.; Xu, J. Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against β-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem. Biol. Interact. 2009, 181, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Liu, Y.; Shi, M.; Liu, X.; Li, L.; Liu, Y.; Zhao, G. Promotive effect of ginsenoside Rd on proliferation of neural stem cells in vivo and in vitro. J. Ethnopharmacol. 2012, 42, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Tchantchou, F.; Xu, Y.; Wu, Y.; Christen, Y.; Luo, Y. EGb761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2016, 21, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, Z.; Butko, P.; Christen, Y.; Lambert, M.; Klein, W.; Link, C.; Luo, Y. Amyloid-β-Induced Pathological Behaviors Are Suppressed by Ginkgo biloba Extract EGb761 and Ginkgolides in Transgenic Caenorhabditis elegans. J. Neurosci. 2006, 26, 13102–13113. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cui, C.; Pang, C.; Christen, Y.; Luo, Y. Restoration of impaired phosphorylation of cyclic AMP response element-binding protein (CREB) by EGb761 and its constituents in Aβ-expressing neuroblastoma cells. Eur. J. Neurosci. 2007, 26, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.; Khodagholi, F. Natural Products as Promising Drug Candidates for the Treatment of Alzheimer’s Disease: Molecular Mechanism Aspect. Curr. Neuropharmacol. 2013, 11, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-Y.; Lee, Y.-J.; Hong, J.T.; Lee, H.-J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.L.; Choi, R.C.; Zhu, K.Y.; Leung, K.-W.; Guo, A.J.; Bi, D.; Xu, H.; Lau, D.T.; Dong, T.T.; Tsim, K.W. Isorhamnetin, A Flavonol Aglycone from Ginkgo biloba L., Induces Neuronal Differentiation of Cultured PC12 Cells: Potentiating the Effect of Nerve Growth Factor. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2015, 10, 23–42. [Google Scholar]
- Jia, S.-L.; Wu, X.-L.; Li, X.-X.; Dai, X.-L.; Gao, Z.-L.; Lu, Z.; Zheng, Q.-S.; Sun, Y.-X. Neuroprotective effects of liquiritin on cognitive deficits induced by soluble amyloid-β1-42 oligomers injected into the hippocampus. J. Asian Nat. Prod. Res. 2016, 18, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-A.; Wang, J.-L.; Liu, R.-T.; Ren, J.-P.; Wen, L.-Q.; Chen, X.-J.; Bian, G.-X. Liquiritin potentiate neurite outgrowth induced by nerve growth factor in PC12 cells. Cytotechnology 2009, 60, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-H.; Chang, T.-Y.; Ko, W.-C. Interaction Between Daidzein and Hesperetin on Antispasmodic Action in Isolated Sensitized and Non-sensitized Guinea-Pig Tracheas. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jeng, C.; Chien, C.; Wang, S. Signaling mechanisms of daidzein-induced axonal outgrowth in hippocampal neurons. Biochem. Biophys. Res. Commun. 2008, 366, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, Q.; Diaz, R. Neuroprotective and neurotorphic efficacy of phytoestrogens in cultured hippocampal neurons. Exp. Biol. Med. 2002, 227, 509–519. [Google Scholar]
- Yang, S.-H.; Liao, C.-C.; Chen, Y.; Syu, J.-P.; Jeng, C.-J.; Wang, S.-M. Daidzein induces neuritogenesis in DRG neuronal cultures. J. Biomed. Sci. 2012, 19. [Google Scholar] [CrossRef] [PubMed]
- Benes, C.; Soltoff, S. Modulation of PKCδ tyrosine phosphorylation and activity in salivary and PC-12 cells by Src kinases. Am. J. Physiol. Cell Physiol. 2001, 280, C1498–C1510. [Google Scholar] [PubMed]
- Tohda, C.; Tamura, T.; Matsuyama, S.; Komatsu, K. Promotion of axonal maturation and prevention of memory loss in mice by extracts of Astragalus mongholicus. Br. J. Pharmacol. 2006, 149, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Thomas, P.; Zhong, J.-H.; Bi, F.-F.; Kosaraju, S.; Pollard, A.; Fenech, M.; Zhou, X.-F. Consumption of Grape Seed Extract Prevents Amyloid-β Deposition and Attenuates Inflammation in Brain of an Alzheimer’s Disease Mouse. Neurotoxicol. Res. 2009, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br. J. Clin. Pharmacol. 2013, 75, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Wang, Y.-J.; Zhong, J.-H.; Kosaraju, S.; O’Caliaghan, N.; Zhou, X.-F.; Fenech, M. Grape seed polyphenols and curcumin reduce genomic instability events in a transgenic mouse model for Alzheimer’s disease. Mutat. Res. 2009, 661, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ferruzzi, M.; Ho, L.; Blount, J.; Janle, E.; Gong, B.; Pan, Y.; Gowda, N.; Raftery, D.; Arrieta-Cruz, I.; et al. Brain-Targeted Proanthocyanidin Metabolites for Alzheimer’s Disease Treatment. J. Neurosci. 2012, 32, 5144–5150. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.-M.; Lin, J.-Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, L.; Yang, H.; Li, C.; Hui, Z.; Xu, Y.; Zhu, X. Oridonin Attenuates Synaptic Loss and Cognitive Deficits in an Aβ1–42-Induced Mouse Model of Alzheimer’s Disease. PLoS ONE 2016, 11, e0151397. [Google Scholar] [CrossRef] [PubMed]
- Nakagawasai, O.; Yamadera, F.; Iwasaki, K.; Arai, H.; Taniguchi, R.; Tan-No, K.; Sasaki, H.; Tadano, T. Effect of kami-untan-to on the impairment of learning and memory induced by thiamine-deficient feeding in mice. Neuroscience 2004, 125, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.G.; Kim, D.H.; Park, S.J.; Kim, J.M.; Cai, M.; Liu, X.; Lee, C.H.; Ryu, J.H. The memory-enhancing effects of Kami-ondam-tang in mice. J. Ethnopharmacol. 2011, 137, 251–256. [Google Scholar]
- Ren, Z.L.; Zuo, P.P. Neural Regeneration: Role of Traditional Chinese Medicine in Neurological Diseases Treatment. J. Pharmacol. Sci. 2012, 120, 139–145. [Google Scholar]
- Yang, H.; Wen, S.R.; Zhang, G.W.; Wang, T.G.; Hu, F.X.; Li, X.L.; Wang, D.S. Effects of Chinese herbal medicine Fuzhisan on autologous neural stem cells in the brain of SAMP-8 mice. Exp. Gerontol. 2011, 46, 628–636. [Google Scholar]
- Fu, X.; Wang, Q.; Wang, Z.; Kuang, H.; Jiang, P. Danggui-Shaoyao-San: New Hope for Alzheimer’s Disease. Aging Dis. 2016, 7, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Mao, Q.Q.; Zhong, X.M.; Li, Z.Y.; Qiu, F.M.; Ip, S.P. Mechanistic Study on the Antidepressant-Like Effect of Danggui-Shaoyao-San, a Chinese Herbal Formula. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, H.; Yao, W.; Gao, X. Aqueous extract of the Chinese medicine, Danggui-Shaoyao-San, inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventingcytochrome c release and inactivating of caspase cascade. Cell Biol. Int. 2008, 32, 304–311. [Google Scholar] [PubMed]
- Kou, J.; Zhu, D.; Yan, Y. Neuroprotective effects of the aqueous extract of the Chinese medicine Danggui-Shaoyao-San on aged mice. J. Ethnopharmacol. 2005, 97, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Wang, B.; Li, N.; Jin, K.; Ji, X. Herbal Formula Danggui-Shaoyao-San Promotes Neurogenesis and Angiogenesis in Rat Following Middle Cerebral Artery Occlusion. Aging Dis. 2015, 6, 245–253. [Google Scholar] [CrossRef] [PubMed]




| No. | Active Ingredients | Herb Source | Class | Modes of Delivery In Vivo/In Vitro | Assay (In Vivo/In Vitro) | Observations | Possible Mechanisms | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Curcumin | Curcuma longa | Polyphenol | Intraperitoneal | In vivo: Conditioned Avoidance test In vitro: Neurosphere assay, Luciferase reporter assay | Increased p-GSK-3β, Reelin and Pax6 in the hippocampus | Canonical Wnt/β-catenin pathway | [50] |
| 2 | Huperzine A | Huperzia serrata | Alkaloid | Intraperitoneal | In vitro: CCK-8 cell proliferation assay | Neural stem cell proliferation | MEK pathway | [51] |
| 3 | Ginsenosides | Panax ginseng | Glycoside | Intraperitoneal | In vivo: BrdU administration In vitro: VEGF and BDNF assay | Increased VEGF and BDNF | AKT and ERK pathway | [52] |
| 4 | Ginkgolide | Ginkgo biloba | Prenol | Oral | In vivo: BrdU administration In vitro: Immunoblotting | Inhibition of Aβ oligomerization | BDNF/CREB pathway | [53] |
| 5 | Salvianolic acid B | Salvia miltiorrhiza | Phenolic acid | In vitro cell culture | In vitro: Neurosphere culture, RT-PCR, Immunofluorescence | Decreased LDH leakage, inhibition of apoptosis | BDNF activation | [54] |
| 6 | Asarone | Acori tatarinowii | Phenyl proponoid | Intraperitoneal | In vivo: BrdU administration In vitro: Immunoblotting | NPC proliferation, Neurogenesis | MEK pathway | [55] |
| 7 | Epicatechin | Camellia sinensis | Flavanoid | Intraperitonieal | In vivo: BrdU administration In vitro: Immunoblotting | Elevated BDNF in hippocampus | Increase Tyrosine hydroxylase levels in brain | [56] |
| 8 | Oridonin | Rabdosia rubescens | Terpenoid | Intraperitonieal | In vivo: Morris water maze. In vitro: Synaptosomal activity assay, Golgi staining | Increase in PSD95, Synaptophysin, BDNF, p-TrkB and p-CREB | BDNF/TrkB/CREB pathway | [57] |
| 9 | Catalpol | Rehmannia glutinosa | Iridoid | Oral | In vivo: Y-maze. In vitro: AChE activity, Radio-ligand binding assay, ChAT activity | Increase in M-receptor density and neuritic outgrowth | BDNF/TrkB/CREB pathway | [58] |
| 10 | Senegenin | Polygala tenuifolia | Iridoid | In vitro cell culture | In vitro: PC12 neurite outgrowth experiment | Neuronal outgrowth, Increase in GAP-43 and MAP2 | PKC pathway | [59] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreenivasmurthy, S.G.; Liu, J.-Y.; Song, J.-X.; Yang, C.-B.; Malampati, S.; Wang, Z.-Y.; Huang, Y.-Y.; Li, M. Neurogenic Traditional Chinese Medicine as a Promising Strategy for the Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 18, 272. https://doi.org/10.3390/ijms18020272
Sreenivasmurthy SG, Liu J-Y, Song J-X, Yang C-B, Malampati S, Wang Z-Y, Huang Y-Y, Li M. Neurogenic Traditional Chinese Medicine as a Promising Strategy for the Treatment of Alzheimer’s Disease. International Journal of Molecular Sciences. 2017; 18(2):272. https://doi.org/10.3390/ijms18020272
Chicago/Turabian StyleSreenivasmurthy, Sravan Gopalkrishnashetty, Jing-Yi Liu, Ju-Xian Song, Chuan-Bin Yang, Sandeep Malampati, Zi-Ying Wang, Ying-Yu Huang, and Min Li. 2017. "Neurogenic Traditional Chinese Medicine as a Promising Strategy for the Treatment of Alzheimer’s Disease" International Journal of Molecular Sciences 18, no. 2: 272. https://doi.org/10.3390/ijms18020272
APA StyleSreenivasmurthy, S. G., Liu, J. -Y., Song, J. -X., Yang, C. -B., Malampati, S., Wang, Z. -Y., Huang, Y. -Y., & Li, M. (2017). Neurogenic Traditional Chinese Medicine as a Promising Strategy for the Treatment of Alzheimer’s Disease. International Journal of Molecular Sciences, 18(2), 272. https://doi.org/10.3390/ijms18020272